
Concept explainers
The first stage of treatment at the reverse osmosis plant in Carlsbad, California, is to flow the water through rock, sand, and gravel as shown here. Would this step remove particulate matter? Would this step remove dissolved salts? [Section 18.4]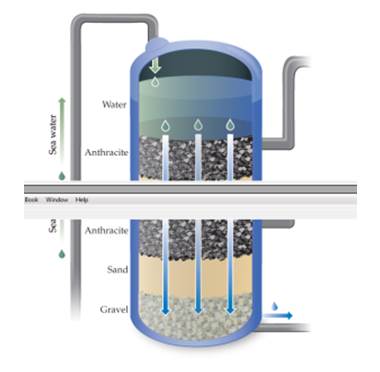
Learn your wayIncludes step-by-step video

Chapter 4 Solutions
Chemistry: The Central Science, Books a la Carte Edition & Solutions to Red Exercises for Chemistry & Mastering Chemistry with Pearson eText -- Access Card Package
Additional Science Textbook Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Concepts of Genetics (12th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Chemistry: A Molecular Approach (4th Edition)
Microbiology: An Introduction
- Please answer the question and provide a detailed drawing of the structure. If there will not be a new C – C bond, then the box under the drawing area will be checked. Will the following reaction make a molecule with a new C – C bond as its major product: Draw the major organic product or products, if the reaction will work. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry.arrow_forwardPlease do not use AI. AI cannot "see" the molecules properly, and it therefore gives the wrong answer while giving incorrect descriptions of the visual images we're looking at. All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forwardPlease answer the question and provide detailed explanations.arrow_forward
- All of these compounds would be produced (I think). In my book, I don't see any rules about yield in this case, like explaining that one product would be present in less yield for this reason or that reason. Please explain why some of these produce less yield than others.arrow_forward5. Fill in the missing molecules in the following reaction pathway. TMSO Heat + CI then HF O₂N (1.0 equiv) AICI 3 OMearrow_forwarde. O₂N NO2 1. excess H2, Pd/C 2. excess NaNO2, HCI 3. excess CuCNarrow_forward
- Help with a periodic table task.' Procedure Part 1: Customizing a Periodic Table Use a textbook or other valid source to determine which elements are metals, nonmetals, metalloids (called semimetals in some texts), alkali metals, alkaline earth metals, transition metals, halogens, and noble gases. Download and print a copy of the Periodic Table of Elements. Use colored pencils, colorful highlighters, or computer drawing tools to devise a schematic for designating each of the following on the periodic table: Group numbers Period number Labels for these groups: alkali metals, alkaline earth metals, transition metals, inner transition metals (lanthanides and actinides), other metals, metalloids (semimetals), other nonmetals, halogens, and noble gases Metals, nonmetals, and metalloids Note: Write the group and period numbers and color/highlight each element for categorization. Be sure to include a key for the schematic. Take a photo of the completed periodic table and upload the…arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardCan you explain these two problems for mearrow_forward
- 个 ^ Blackboard x Organic Chemistry II Lecture (m x Aktiv Learning App x → C app.aktiv.com ← Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 28 of 35 :OH H HH KO Select to Edit Arrows CH CH₂OK, CH CH2OH 5+ H :0: Donearrow_forwardCan you explain those two problems for me please.arrow_forwardDo we need to draw the "ethyne" first for this problem? im confusedarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER





