
Chemistry: The Central Science, Books a la Carte Edition & Modified Mastering Chemistry with Pearson eText -- ValuePack Access Card Package
1st Edition
ISBN: 9780133910919
Author: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.5, Problem 4.14.1PE
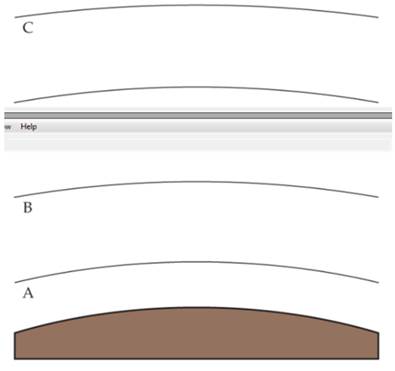
The figure shows the three lowest regions of Earth’s atmosphere.
- Name each and indicate the approximate elevations at which the boundaries occur.
- In which region is ozone a pollutant? In which region does it filter UV solar radiation?
- In which region in infrared radiation from Earth’s surface most strongly reflected back?
- An aurora borealis is due to excitation of atoms and molecules in the atmosphere 55-95 km above Earth’s surface. Which regions in the figure are involved in an aurora borealis?
- Compare the changes in relative concentrations of water vapor and carbon dioxide with increasing elevation in these three regions [Section 18.1].
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Redraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately
represent the direction of the bonds to ring substituents.
Cl.
Br
Click and drag to start drawing a
structure.
: ☐
☑
P
K
m
Choose the best reagents to complete the following reaction.
L
ZI
0
Problem 4 of 11
A
1. NaOH
2. CH3CH2CH2NH2
1. HCI
B
OH
2. CH3CH2CH2NH2
DII
F1
F2
F3
F4
F5
A
F6
C
CH3CH2CH2NH2
1. SOCl2
D
2. CH3CH2CH2NH2
1. CH3CH2CH2NH2
E
2. SOCl2
Done
PrtScn
Home
End
FA
FQ
510
*
PgUp
M
Submit
PgDn
F11
None
Chapter 4 Solutions
Chemistry: The Central Science, Books a la Carte Edition & Modified Mastering Chemistry with Pearson eText -- ValuePack Access Card Package
Ch. 4.1 - Prob. 4.1.1PECh. 4.1 - How are the boundaries between the regions of the...Ch. 4.2 - Air pollution in the Mexico City metropolitan area...Ch. 4.2 - Prob. 4.2.2PECh. 4.2 - Prob. 4.3.1PECh. 4.2 - Prob. 4.3.2PECh. 4.2 - Prob. 4.4.1PECh. 4.2 - Prob. 4.4.2PECh. 4.3 - Distinguish between photodissociation and...Ch. 4.3 - Prob. 4.5.2PE
Ch. 4.3 - Prob. 4.6.1PECh. 4.3 - Prob. 4.6.2PECh. 4.3 - Do the reactions involved in ozone depletion...Ch. 4.3 - Prob. 4.7.2PECh. 4.4 - Prob. 4.8.1PECh. 4.4 - Prob. 4.8.2PECh. 4.4 - Prob. 4.9.1PECh. 4.4 - Prob. 4.9.2PECh. 4.4 - Prob. 4.10.1PECh. 4.4 - Prob. 4.10.2PECh. 4.5 - Practice Exercise 2 The bond energy in N2 is 941...Ch. 4.5 - Prob. 4.11.2PECh. 4.5 - Prob. 4.12.1PECh. 4.5 - Prob. 4.12.2PECh. 4.5 - Prob. 4.13.1PECh. 4.5 - Prob. 4.13.2PECh. 4.5 - The figure shows the three lowest regions of...Ch. 4.5 - Prob. 4.14.2PECh. 4.6 - Where does the energy come from to evaporate the...Ch. 4.6 - Prob. 4.15.2PECh. 4.6 - Prob. 4.16.1PECh. 4.6 - The first stage of treatment at the reverse...Ch. 4.6 - Prob. 4.17.1PECh. 4.6 - Prob. 4.17.2PECh. 4 - Prob. 1DECh. 4 - Prob. 1ECh. 4 - Prob. 2ECh. 4 - Prob. 3ECh. 4 - Prob. 4ECh. 4 - Prob. 5ECh. 4 - Prob. 6ECh. 4 - Which of the following ions will always be a...Ch. 4 - Prob. 8ECh. 4 - Prob. 9ECh. 4 - Prob. 10ECh. 4 - Prob. 11ECh. 4 - List the common products formed when an organic...Ch. 4 - Prob. 13ECh. 4 - Prob. 14ECh. 4 - Prob. 15ECh. 4 - Prob. 16ECh. 4 - Prob. 17ECh. 4 - Prob. 18ECh. 4 - Prob. 19ECh. 4 - Prob. 20ECh. 4 - Prob. 21ECh. 4 - Prob. 22ECh. 4 - Prob. 23ECh. 4 - Prob. 24ECh. 4 - Prob. 25ECh. 4 - Prob. 26ECh. 4 - Prob. 27ECh. 4 - Prob. 28ECh. 4 - Prob. 29ECh. 4 - Explain, using Le Châtelier’s principle, why the...Ch. 4 - Prob. 31ECh. 4 - Prob. 32ECh. 4 - Prob. 33ECh. 4 - Prob. 34ECh. 4 - Prob. 35ECh. 4 - Prob. 36ECh. 4 - Prob. 37ECh. 4 - Prob. 38ECh. 4 - Prob. 39ECh. 4 - Prob. 40ECh. 4 - Prob. 41ECh. 4 - Prob. 42ECh. 4 - Prob. 43ECh. 4 - Prob. 44ECh. 4 - Prob. 45ECh. 4 - 18.85 The main reason that distillation is a...Ch. 4 - Prob. 47ECh. 4 - Prob. 48ECh. 4 - Prob. 49ECh. 4 - Prob. 50ECh. 4 - Prob. 51ECh. 4 - Prob. 52ECh. 4 - The process of iron being oxidized to make iron...Ch. 4 - At 1 atm pressure, CO2(s) sublimes at 78oC. Is...Ch. 4 - Prob. 55ECh. 4 - Prob. 56ECh. 4 - Prob. 57ECh. 4 - Prob. 58ECh. 4 - Prob. 59ECh. 4 - Prob. 60ECh. 4 - Using the standard molar entropies in Appendix C,...Ch. 4 - Which of these statements is true? All spontaneous...Ch. 4 - Prob. 63ECh. 4 - Prob. 64ECh. 4 - Prob. 65ECh. 4 - Prob. 66ECh. 4 - Prob. 67ECh. 4 - What is the temperature above which the Haber...Ch. 4 - Prob. 69ECh. 4 - Prob. 70ECh. 4 - Prob. 71ECh. 4 - Prob. 72ECh. 4 - Prob. 73ECh. 4 - Prob. 74ECh. 4 - Prob. 75ECh. 4 - Prob. 76ECh. 4 - As shown here, one type of computer keyboard...Ch. 4 - 19.3
a. What are the signs of ΔS and ΔH for the...Ch. 4 - Predict the signs of H and S for this reaction....Ch. 4 - Prob. 80ECh. 4 - The accompanying diagram shows how H (red line)...Ch. 4 - Prob. 82ECh. 4 - Prob. 83ECh. 4 - Prob. 84ECh. 4 - Prob. 85ECh. 4 - Prob. 86ECh. 4 - Prob. 87ECh. 4 - Can endothermic chemical reaction be spontaneous?...Ch. 4 - Prob. 89ECh. 4 - Prob. 90ECh. 4 - Prob. 91AECh. 4 - Prob. 92AECh. 4 - Prob. 93AECh. 4 - Prob. 94AECh. 4 - Prob. 95AECh. 4 - Prob. 96AECh. 4 - Prob. 97AECh. 4 - Prob. 98AECh. 4 - Prob. 99AECh. 4 - Prob. 100AECh. 4 - Prob. 101AECh. 4 - Prob. 102AECh. 4 - Prob. 103AECh. 4 - Alcohol-based fuels for automobiles lead to the...Ch. 4 - Prob. 105IECh. 4 - Prob. 106IECh. 4 - Prob. 107IECh. 4 - Prob. 108IECh. 4 - Prob. 109IECh. 4 - Prob. 110IECh. 4 - Prob. 111IECh. 4 - Prob. 112IECh. 4 - Although there are many ions in seawater, the...Ch. 4 - The Ogallala aquifer described in the Close Look...Ch. 4 - Prob. 115IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Add curved arrows to the reactants in this reaction. A double-barbed curved arrow is used to represent the movement of a pair of electrons. Draw curved arrows. : 0: si H : OH :: H―0: Harrow_forwardConsider this step in a radical reaction: Br N O hv What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. O primary Otermination O initialization O electrophilic O none of the above × ☑arrow_forwardNonearrow_forward
- Can I get a drawing of what is happening with the orbitals (particularly the p orbital) on the O in the OH group? Is the p orbital on the O involved in the ring resonance? Why or why not?arrow_forward1) How many monochlorination products-including stereochemistry- are there for the molecule below:arrow_forwardSelect an amino acid that has and N-H or O-H bond in its R-group (you have 8 to choose from!). Draw at least two water molecules interacting with the R-group of the amino acid.arrow_forward
- Is this aromatic?arrow_forwardCHEM2323 E Tt PS CH03 Draw and name all monobromo derivatives of pentane, C5H11Br. Problem 3-33 Name: Draw structures for the following: (a) 2-Methylheptane (d) 2,4,4-Trimethylheptane Problem 3-35 (b) 4-Ethyl-2,2-dimethylhexane (e) 3,3-Diethyl-2,5-dimethylnonane (c) 4-Ethyl-3,4-dimethyloctane 2 (f) 4-Isopropyl-3-methylheptane KNIE>arrow_forwardProblem 3-42 Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. Problem 3-44 Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2- dibromoethane. Problem 3-45 Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the actual conformation of the molecule?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY