
Concept explainers
(a)
The initial temperature of the piston cylinder device.
The final temperature of the piston cylinder device.
(a)
Answer to Problem 114RP
The initial temperature of the piston cylinder device is
The final temperature of the piston cylinder device is
Explanation of Solution
Determine the total initial volume of piston cylinder device.
Here, the mass of the liquid phase is
Determine the total volume of the piston cylinder device at final state.
Determine the specific volume of the piston cylinder device at final state.
Here, the mass of the saturated liquid vapour mixture of water is contained in a piston cylinder device is
Conclusion:
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is saturated pressure and saturated temperature.
For initial temperature of the piston cylinder device.
Show the temperature at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (1).
|
Pressure, kPa |
Temperature, C |
| 150 kPa | 111.35 |
| 160 kPa | |
| 175 kPa | 116.04 |
Substitute the value of x and y from Table (1) in Equation (IV) to calculate the value of initial temperature
Thus, the initial temperature of the piston cylinder device is
For specific volume of saturated liquid of the piston cylinder device.
Show the specific volume of saturated liquid at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (2).
|
Pressure, kPa |
Specific volume of saturated liquid, |
| 150 kPa | 0.001053 |
| 160 kPa | |
| 175 kPa | 0.001057 |
Substitute the value of x and y from Table (2) in Equation (IV) to calculate the value of specific volume of saturated liquid
For specific volume of saturated vapour of the piston cylinder device.
Show the specific volume of saturated vapour at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (3).
|
Pressure, kPa |
Specific volume of saturated vapour, |
| 150 kPa | 1.1594 |
| 160 kPa | |
| 175 kPa | 1.0037 |
Substitute the value of x and y from Table (3) in Equation (IV) to calculate the value of specific volume of saturated vapour
Substitute
Substitute
Substitute
The unit conversion of pressure from kPa to MPa.
For temperature of the piston cylinder device at final state.
Show the temperature at specific volume of the piston cylinder device at final state at
|
specific volume of the piston cylinder device at final state, |
Temperature, |
| 600 | |
| 700 |
Substitute the value of x and y from Table (4) in Equation (IV) to calculate the value of temperature of the piston cylinder device at final state
Thus, the final temperature of the piston cylinder device is
(b)
The mass of liquid water when the piston first starts moving.
(b)
Answer to Problem 114RP
The mass of liquid water when the piston first starts moving is
Explanation of Solution
Determine the specific volume of the piston cylinder device at this state.
Here, the mass of the saturated liquid vapour mixture of water is contained in a piston cylinder device is
Conclusion:
Since,
Substitute
Therefore, the value of specific volume of the piston cylinder device at this state is greater than
Thus, the mass of liquid water when the piston first starts moving is
(c)
The work done during the process state 2 and 3.
(c)
Answer to Problem 114RP
The work done during the process state 2 and 3 is
Explanation of Solution
Determine the work done in constant pressure process.
Conclusion:
Substitute
Thus, the work done during the process state 2 and 3 is
Show the P-v diagram of this process.
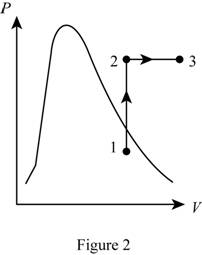
Want to see more full solutions like this?
Chapter 4 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- (read image) Answer:arrow_forwardThe correct answer is ~168 MPa, how was this found?arrow_forwardAir enters the compressor of a regenerative gas turbine engine at 310 K and 100 kPa, where it is compressed to 900 kPa and 650 K. The regenerator has an effectiveness of 75%, and the air enters the turbine at 1400 K. Assume variable specific heats for air. For a turbine efficiency of 90 percent, determine the amount of heat transfer in the regenerator. The amount of heat transfer in the regenerator is kJ/kg.arrow_forward
- Air enters the compressor of a regenerative gas turbine engine at 310 K and 100 kPa, where it is compressed to 900 kPa and 650 K. The regenerator has an effectiveness of 79 percent, and the air enters the turbine at 1400 K. Assume constant specific heats for air at room temperature. The properties of air at room temperature are cp = 1.005 kJ/kg·K and k = 1.4. For a turbine efficiency of 90 percent, determine the amount of heat transfer in the regenerator. The amount of heat transfer in the regenerator is kJ/kg.arrow_forwardHints: Find the closed loop transfer function and then plot the step response for diFerentvalues of K in MATLAB. Show step response plot for different values of K. Auto Controls Show solutions and provide matlab code NO COPIED ANSWERS OR WILL REPORT!!!! Use own solutionarrow_forwardwhat is shear stress and normal? how to tell them while calculating?arrow_forward
- 12 mm 45 mm 20 kN 20 kN 12 mm 45 mm PROBLEM 1.61 For the assembly and loading of Problem 1.60, determine (a) the average shearing stress in the pin at C, (b) the average bearing stress at C in member BC, (c) the average bearing stress at B in member BC. PROBLEM 1.60 Two horizontal 20-kN forces are applied to pin B of the assembly shown. Knowing that a pin of 20-mm diameter is used at each connection, determine the maximum value of the average normal stress (a) in link AB, (b) in link BC.arrow_forwardHow do you find these answers?arrow_forward250 mm 400 mm A B C E F 250 mm PROBLEM 1.52 Each of the two vertical links CF connecting the two horizontal members AD and EG has a 10 × 40-mm uniform rectangular cross section and is made of a steel with an ultimate strength in tension of 400 MPa, while each of the pins at C and F has a 20-mm diameter and are made of a steel with an ultimate strength in shear of 150 MPa. Determine the overall factor of safety for the links CF and the pins connecting them to the horizontal members. 24 kNarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





