
(a) In an HCl molecule, take the Cl atom to be the isotope 35Cl. The equilibrium separation of the H and Cl atoms is 0.127 46 nm. The
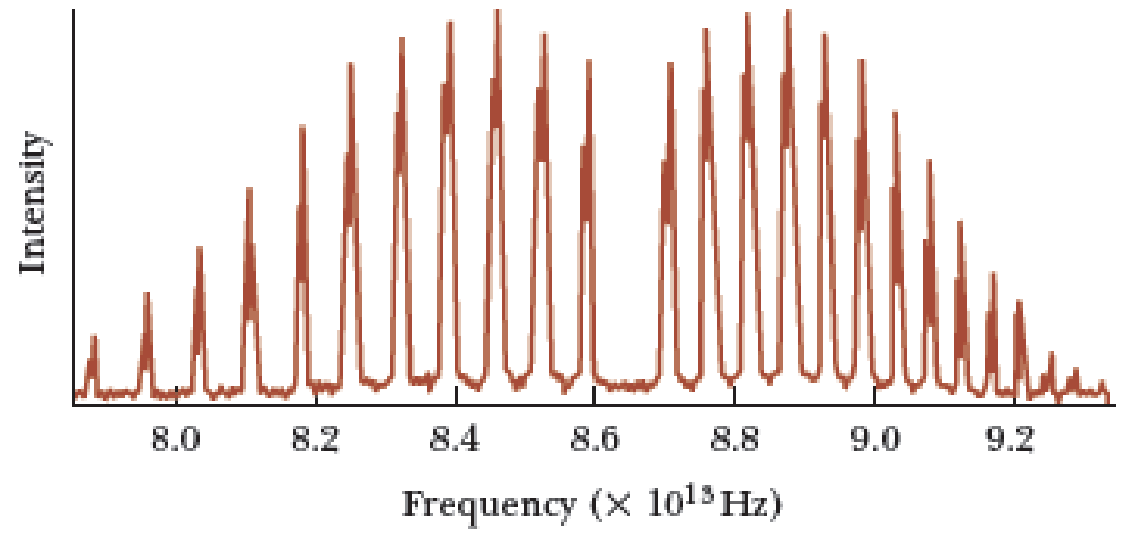
(a)
The longest wavelength in the rotational spectrum of HCl molecule when
Answer to Problem 11P
The longest wavelength in the rotational spectrum of
Explanation of Solution
Write the equation for the smallest energy difference between the rotational energy levels.
Here,
Write the equation for the wavelength.
Here,
Substitute equation (I) in the equation (II) and substitute
Write the equation for the reduced mass.
Here,
Write the equation for moment of inertia.
Here,
Conclusion:
Substitute equation (V) in equation (III).
Substitute
Substitute
Here,
Substitute
Thus, the longest wavelength in the rotational spectrum of HCl molecule when
(b)
The longest wavelength in the rotational spectrum of HCl molecule when
Answer to Problem 11P
The longest wavelength in the rotational spectrum of HCl molecule when
Explanation of Solution
From the equation (IV) for the reduced mass.
From the equation (VI) for wavelength.
Conclusion:
Substitute
Here,
Substitute
Thus, the longest wavelength in the rotational spectrum of HCl molecule when
(c)
The separation in wavelength between the doublet lines for the longest wavelength.
Answer to Problem 11P
The separation in wavelength between the doublet lines for the longest wavelength is
Explanation of Solution
Write the equation for the separation in wavelength between the doublet lines for the longest wavelength.
Here,
Conclusion:
Substitute
Thus, the separation in wavelength between the doublet lines for the longest wavelength is
Want to see more full solutions like this?
Chapter 42 Solutions
Physics for Scientists and Engineers with Modern Physics
- Q: What is the direction of the magnetic field at point A, due to the current I in a wire, in each of the cases 1 to 6 shown below? Note: point A is in the plane of the page. ▪A I I ▪A (1) (2) ▪A • I (out of page) (3) ▪A I x I (into page) ▪A ▪A I (4) (5) (6)arrow_forwardA tennis ball is thrown into the air with initial speed vo=46 m/s and angle (theta) 38 degrees from the ground. Find the distance it travels (x) when it hits the ground.arrow_forwardProblem 04.08 (17 points). Answer the following questions related to the figure below. ථි R₁ www R₂ E R₁ www ли R₁ A Use Kirchhoff's laws to calculate the currents through each battery and resistor in terms of R1, R2, E1, & E2. B Given that all the resistances and EMFs have positive values, if E₁ > E2 and R₁ > R2, which direction is the current flowing through E₁? Through R₂? C If E1 E2 and R₁ > R2, which direction is the current flowing through E₁? Through R2?arrow_forward
- A 105- and a 45.0-Q resistor are connected in parallel. When this combination is connected across a battery, the current delivered by the battery is 0.268 A. When the 45.0-resistor is disconnected, the current from the battery drops to 0.0840 A. Determine (a) the emf and (b) the internal resistance of the battery. 10 R2 R₁ ww R₁ Emf 14 Emf Final circuit Initial circuitarrow_forwardA ball is shot at an angle of 60° with the ground. What should be the initial velocity of the ball so that it will go inside the ring 8 meters away and 3 meters high. Suppose that you want the ball to be scored exactly at the buzzer, determine the required time to throw and shoot the ball. Full solution and figure if there is.arrow_forwardCorrect answer please. I will upvote.arrow_forward
- Define operational amplifierarrow_forwardA bungee jumper plans to bungee jump from a bridge 64.0 m above the ground. He plans to use a uniform elastic cord, tied to a harness around his body, to stop his fall at a point 6.00 m above the water. Model his body as a particle and the cord as having negligible mass and obeying Hooke's law. In a preliminary test he finds that when hanging at rest from a 5.00 m length of the cord, his body weight stretches it by 1.55 m. He will drop from rest at the point where the top end of a longer section of the cord is attached to the bridge. (a) What length of cord should he use? Use subscripts 1 and 2 respectively to represent the 5.00 m test length and the actual jump length. Use Hooke's law F = KAL and the fact that the change in length AL for a given force is proportional the length L (AL = CL), to determine the force constant for the test case and for the jump case. Use conservation of mechanical energy to determine the length of the rope. m (b) What maximum acceleration will he…arrow_forward9 V 300 Ω www 100 Ω 200 Ω www 400 Ω 500 Ω www 600 Ω ww 700 Ω Figure 1: Circuit symbols for a variety of useful circuit elements Problem 04.07 (17 points). Answer the following questions related to the figure below. A What is the equivalent resistance of the network of resistors in the circuit below? B If the battery has an EMF of 9V and is considered as an ideal batter (internal resistance is zero), how much current flows through it in this circuit? C If the 9V EMF battery has an internal resistance of 2 2, would this current be larger or smaller? By how much? D In the ideal battery case, calculate the current through and the voltage across each resistor in the circuit.arrow_forward
- helparrow_forwardIf the block does reach point B, how far up the curved portion of the track does it reach, and if it does not, how far short of point B does the block come to a stop? (Enter your answer in m.)arrow_forwardTruck suspensions often have "helper springs" that engage at high loads. One such arrangement is a leaf spring with a helper coil spring mounted on the axle, as shown in the figure below. When the main leaf spring is compressed by distance yo, the helper spring engages and then helps to support any additional load. Suppose the leaf spring constant is 5.05 × 105 N/m, the helper spring constant is 3.50 × 105 N/m, and y = 0.500 m. Truck body yo Main leaf spring -"Helper" spring Axle (a) What is the compression of the leaf spring for a load of 6.00 × 105 N? Your response differs from the correct answer by more than 10%. Double check your calculations. m (b) How much work is done in compressing the springs? ☑ Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. Jarrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





