
(a)
Interpretation:
The systematic name for the following compound should be determined.
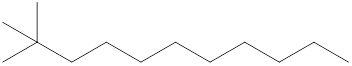
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The systematic name for the following compound should be determined.
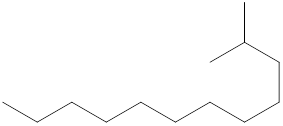
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(c)
Interpretation:
The systematic name for the following compound should be determined.
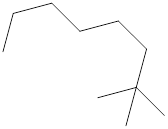
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The systematic name for the following compound should be determined.
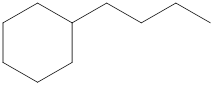
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY-PRINT MULTI TERM
- When N,N-dimethylaniline is treated with bromine both the ortho and para products are observed. However when treated with a mixture of nitric acid and sulfuric acid only the meta product is observed. Explain these results and support your answer with the appropriate drawings *Hint amines are bases* N HNO3 H2SO4 N NO2 N Br2 N Br + N 8-8-8 FeBr3 Brarrow_forwardDraw a mechanism that explains the formation of compound OMe SO3H 1. Fuming H2SO4arrow_forwardConsider the following two acid-base reactions: OH OHI Based on what you know about the compounds and their acidity, which direction would you expect both of these reactions to proceed? Show your reasoning. A pKa table has been provided in case you need it. Functional group Example pka CHA -50 Alkane -35 Amine : NH3 Alkyne RH 25 Water HO-H 169 16 10 Protonated amines NH 10 5 Carboxylic acids OH Hydrochloric acid HCI A chemist intends to run the following reaction on the three substrates shown below: H₂O R-CI product room temp. Cl Cl (1) (2) (3) They find one will react quickly, one slowly, and one will not react at all. Which is which, and why? HINT: What is the reaction they're trying to do? Does that mechanism tell you anything about why something would be favored?arrow_forward
- NH3 decomposes through an equilibrium reaction between NH3, H2, and N2. Only one of the options is correct:(A). The mechanism of the NH3 decomposition reaction must necessarily involve the collision of two NH3 molecules to induce a rearrangement of the atoms in this molecule.(B). The molecular weight of the NH3 decomposition reaction is 2 since two NH3 molecules must collide.(C). The rate of the NH3 decomposition reaction must be greater than that of NH3 synthesis, since the former requires two molecules to collide and the latter, four.(D). The NH3 decomposition reaction cannot occur in a single step.arrow_forwardGiven the equilibrium A2 + B2 ⇌ 2 AB where k1 is the rate coefficient of the forward reaction and k-1 is the rate coefficient of the reverse reaction, with the forward reaction being first-order in A2 and B2, and the reverse reaction being second-order in AB. Equilibrium will be reached later if the relative values of the constants are:(A) k1 high and k-1 high(B) k1 high and k-1 low(C) k1 low and k-1 high(D) k1 low and k-1 lowarrow_forwardA 2-step reaction has the following mechanism: | 1. (fast) R2 R+R 2. (slow) R+Q K₂ P k_1 What series does it have? (A). v= - = (k + k1 − k-1)[R2][Q] (B). v=-k₁[R₂] + k₁[R]² - k₂[R][Q] (C). v=k₂[R]²[Q]² (D). v = k[R₂]1/2[Q]arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
- Steps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


