
Concept explainers
(a)
Interpretation: To determine the type of reaction whether it will be an
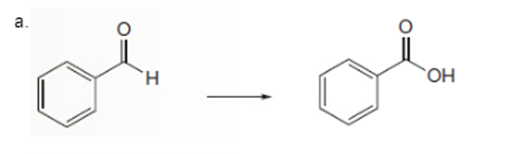
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(b)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
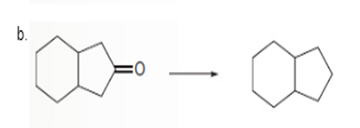
Concept Introduction:
Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(c)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
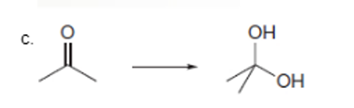
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
(d)
Interpretation: To determine the type of reaction whether it will be an oxidation or reduction reaction.
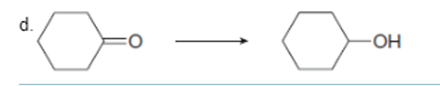
Concept Introduction: Oxidation, which refers to the loss of electrons, is the increase in the oxidation state of its component atoms. When an atom obtains electrons or has its oxidation state reduced, the reduction can occur.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry (6th Edition)
- 4. (3 pts) From the information below, determine the lattice enthalpy for MgBr2. Show all work. AH/(kJ mol-¹) Sublimation of Mg(s) +148 lonization of Mg(g) +2187 to Mg2+(g) Vaporization of Br₂(1) +31 Dissociation of Br,(g) +193 Electron gain by Br(g) -331 Formation of MgBr₂(s) -524arrow_forward1. (4 pts-2 pts each part) Consider the crystal structures of NaCl, ZnS, and CsCl (not necessarily shown in this order). a. For one of the three compounds, justify that the unit cell is consistent with stoichiometry of the compound. b. In each of the crystal structures, the cations reside in certain holes in the anions' packing structures. For each compound, what type of holes are occupied by the cations and explain why those particular types of holes are preferred.arrow_forward(2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward
- (2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward8. (2 pts) Silicon semiconductors have a bandgap of 1.11 eV. What is the longest photon wavelength that can promote an electron from the valence band to the conduction band in a silicon-based photovoltaic solar cell? Show all work. E = hv = hc/λ h = 6.626 x 10-34 Js c = 3.00 x 108 m/s 1 eV 1.602 x 10-19 Jarrow_forwardA solution containing 100.0 mL of 0.155 M EDTA buffered to pH 10.00 was titrated with 100.0 mL of 0.0152 M Hg(ClO4)2 in a cell: calomel electrode (saturated)//titration solution/Hg(l) Given the formation constant of Hg(EDTA)2-, logKf= 21.5, and alphaY4-=0.30, find out the cell voltage E. Hg2+(aq) + 2e- = Hg(l) E0= 0.852 V E' (calomel electrode, saturated KCl) = 0.241 Varrow_forward
- From the following reduction potentials I2 (s) + 2e- = 2I- (aq) E0= 0.535 V I2 (aq) + 2e- = 2I- (aq) E0= 0.620 V I3- (aq) + 2e- = 3I- (aq) E0= 0.535 V a) Calculate the equilibrium constant for I2 (aq) + I- (aq) = I3- (aq). b) Calculate the equilibrium constant for I2 (s) + I- (aq) = I3- (aq). c) Calculate the solubility of I2 (s) in water.arrow_forward2. (3 pts) Consider the unit cell for the spinel compound, CrFe204. How many total particles are in the unit cell? Also, show how the number of particles and their positions are consistent with the CrFe204 stoichiometry - this may or may not be reflected by the particle colors in the diagram. (HINT: In the diagram, the blue particle is in an interior position while each red particle is either in a corner or face position.)arrow_forwardFrom the following potentials, calculate the activity of Cl- in saturated KCl. E0 (calomel electrode)= 0.268 V E (calomel electrode, saturated KCl)= 0.241 Varrow_forward
- Calculate the voltage of each of the following cells. a) Fe(s)/Fe2+ (1.55 x 10-2 M)//Cu2+ (6.55 x 10-3 M)/Cu(s) b) Pt, H2 (0.255 bar)/HCl (4.55 x 10-4 M), AgCl (sat'd)/Ag Fe2+ +2e- = Fe E0= -0.44 V Cu2+ + 2e- = Cu E0= 0.337 V Ag+ + e- = Ag E0= 0.799 V AgCl(s) + e- = Ag(s) + Cl- E0= 0.222 V 2H+ + 2e- = H2 E0= 0.000 Varrow_forwardA solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol). a) Write a balanced net reaction that can occur between species in this solution. b) Calculate deltaG0 and K for the reaction. c) Calculate E and deltaG for the conditions given. Ce4+ + e- = Ce3+ E0= 1.70 V MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 Varrow_forward1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in the following reaction. Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is a weak base used to neutralize strong acid (pKa < 0) produced by the reaction. Redraw the product to show the configuration(s) that form at C-2 and C-4. Br2 OH CH2Cl2 Na* HCO3 Br HO OH + Na Br +arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

