
Write a net ionic equation for any precipitation reaction that occurs when 1 M solutions of the following are mixed.
(a) copper(II) sulfate and sodium chloride
(b) manganese(II) nitrate and ammonium hydroxide
(c) silver nitrate and hydrochloric acid
(d) nickel(II) sulfate and potassium hydroxide
(e) ammonium carbonate and sodium nitrate
(a)
Interpretation:
The net ionic equation should be written when copper(II) sulfate and sodium chloride are mixed.
Concept introduction:
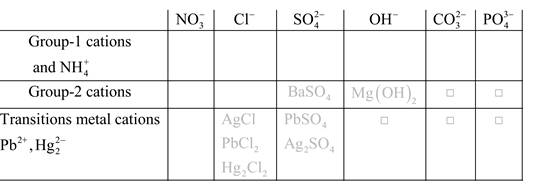
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
No precipitation occurs.
Explanation of Solution
Copper(II) sulfate:
Sodium chloride:
Reaction for the solution of copper(II) sulfate and sodium chloride is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
(b)
Interpretation:
The net ionic equation should be written when manganese(II) nitrate and ammonium hydroxide are mixed.
Concept introduction:
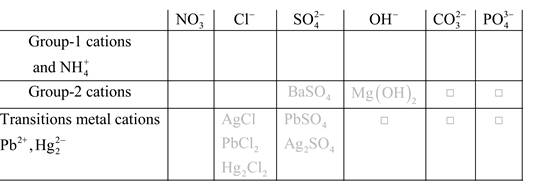
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Manganese(II) nitrate:
Ammonium hydroxide:
Reaction for the solution of manganese(II) nitrate and ammonium hydroxide is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(c)
Interpretation:
The net ionic equation should be written when silver nitrate and hydrochloric acid are mixed.
Concept introduction:
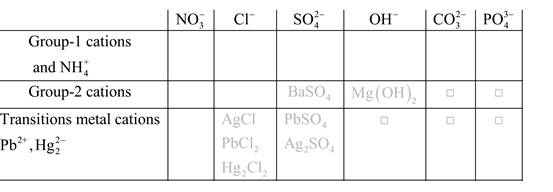
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Silver nitrate:
Hydrochloric acid:
Reaction for the solution of silver nitrate and hydrochloric acid is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(d)
Interpretation:
The net ionic equation should be written when nickel(II) sulfate and potassium hydroxide are mixed.
Concept introduction:
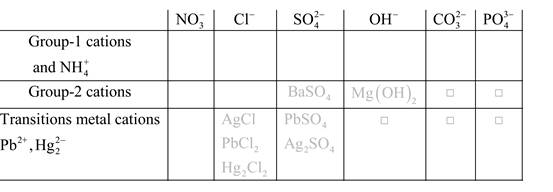
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
Precipitation occurs
The net ionic equation is:
Explanation of Solution
Nickel(II) sulfate:
Potassium hydroxide:
Reaction for the solution of silver nitrate and hydrochloric acid is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
So, the equation will be:
Now, cancelling out the ions which appear on both sides of the equation (
(e)
Interpretation:
The net ionic equation should be written when ammonium carbonate and sodium nitrate are mixed.
Concept introduction:
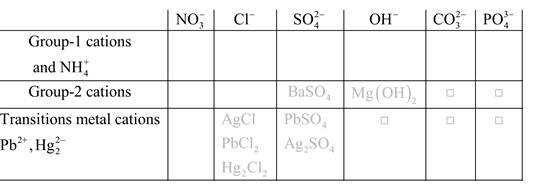
Solubility of any compound is predicted by above solubility chart.
Blank boxes indicate no precipitate formation occurs which means soluble in dilute solution.
Boxes with grey small box will form precipitate from dilute solutions and boxes where formula is written this is a cation-anion combination that will form precipitate.
Precipitation reactions: It is a type of chemical reactions where two soluble salts react with each other and formed different products, out of which one product must be insoluble in solution which is known as precipitate.
A chemical equation which shows only the species that are participated in the reaction is said to be net ionic equation.
Answer to Problem 9QAP
No precipitation occurs.
Explanation of Solution
Ammonium carbonate:
Sodium nitrate:
Reaction for the solution of silver nitrate and hydrochloric acid is written as:
Reactants:
Ions in solution:
Ions in solution:
Products:
Ions in solution:
Ions in solution:
Now,
Want to see more full solutions like this?
Chapter 4 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





