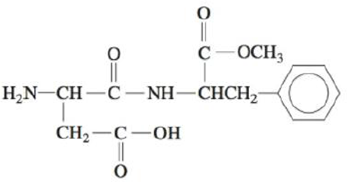
Problem 1RQ: Explain the main postulate of the VSEPR model. List the five base geometries (along with bond... Problem 2RQ: Explain why CF4 and Xef4 are nonpolar compounds (have no net dipole moments) while SF4 is polar (has... Problem 3RQ: Consider the following compounds: CO2, SO2, KrF2, SO3, NF3, IF3, CF4, SF4, XeF4, PF5, TF5, and SCl6.... Problem 4RQ Problem 5RQ: What hybridization is required for central atoms that have a tetrahedral arrangement of electron... Problem 6RQ Problem 7RQ Problem 1ALQ: What are molecular orbitals? How do they compare with atomic orbitals? Can you tell by the shape of... Problem 2ALQ: Explain the difference between the and MOs for homonuclear diatomic molecules. How are bonding and... Problem 3ALQ Problem 4ALQ: Which of the following would you expect to be more favorable energetically? Explain. a. an H2... Problem 5ALQ: Arrange the following molecules from most to least polar and explain your order: CH4, CF2Cl2, CF2H2,... Problem 6ALQ: Which is the more correct statement: The methane molecule (CH4) is a tetrahedral molecule because it... Problem 7ALQ Problem 8ALQ Problem 9Q: Which of the following statements is/are true? Correct the false statements. a. The molecules SeS3.... Problem 10Q: Give one example of a compound having a linear molecular structure that has an overall dipole moment... Problem 11Q: In the hybrid orbital model, compare and contrast bonds with bonds. What orbitals form the bonds... Problem 13Q Problem 14Q Problem 15Q Problem 16Q Problem 17Q: Compare and contrast bonding molecular orbitals with antibonding molecular orbitals. Problem 18Q Problem 19Q: Why does the molecular orbital model do a better job in explaining the bonding in NO and NO than the... Problem 20Q: The three NO bonds in NO3 are all equivalent in length and strength. How is this explained even... Problem 21E: Predict the molecular structure (including bond angles) for each of the following. a. SeO3 b. SeO2 Problem 22E: Predict the molecular structure (including bond angles) for each of the following. a. PCl3 b. SCl2... Problem 23E: Predict the molecular structure and bond angles for each molecule or ion in Exercises 81 and 87 from... Problem 24E Problem 25E Problem 26E: Two variations of the octahedral geometry (see Table 4-1) are illustrated below. Which of the... Problem 27E: Predict the molecular structure (including bond angles) for each of the following. (See Exercises 25... Problem 28E: Predict the molecular structure (including bond angles) for each of the following. (See Exercises 25... Problem 29E: State whether or not each of the following has a permanent dipole moment. a. b. c. d. e. f. Problem 30E: The following electrostatic potential diagrams represent CH4, NH3, or H2O. Label each and explain... Problem 31E: Which of the molecules in Exercises 21 and 22 have net dipole moments (are polar)? Problem 32E: Which of the molecules in Exercises 27 and 28 have net dipole moments (are polar)? Problem 33E: Write Lewis structures and predict the molecular structures of the following. (See Exercises 25 and... Problem 34E: Write Lewis structures and predict whether each of the following is polar or nonpolar. a. HOCN... Problem 35E: Consider the following Lewis structure where E is an unknown element: What are some possible... Problem 36E: Consider the following Lewis structure where E is an unknown element: What are some possible... Problem 37E: The molecules BF3, CF4, CO2, PF5, and SF6 are all nonpolar, even though they all contain polar... Problem 38E: Two different compounds have the formula XeF2Cl2. Write Lewis structures for these two compounds,... Problem 39E: Use the localized electron model to describe the bonding in H2O. Problem 40E: Use the localized electron model to describe the bonding in CCl4. Problem 41E: Use the localized electron model to describe the bonding in H2CO (carbon is the central atom). Problem 42E: Use the localized electron model to describe the bonding in C2H2 (exists as HCCH). Problem 43E: The space-filling models of ethane and ethanol are shown below. Use the localized electron model to... Problem 44E: The space-filling models of hydrogen cyanide and phosgene are shown below. Use the localized... Problem 45E Problem 46E Problem 47E Problem 48E: Give the expected hybridization of the central atom for the molecules in Exercises 27 and 28. Problem 49E: For each of the following molecules, write the Lewis structure(s), predict the molecular structure... Problem 50E: For each of the following molecules or ions that contain sulfur, write the Lewis structure(s),... Problem 51E Problem 52E: The allene molecule has the following Lewis structure: Must all hydrogen atoms lie the same plane?... Problem 53E: Indigo is the dye used in coloring blue jeans. The term navy blue is derived from the use of indigo... Problem 54E Problem 55E Problem 56E: Many important compounds in the chemical industry are derivatives of ethylene (C2H4). two of them... Problem 57E: Two molecules used in the polymer industry are azodicarbonamide and methyl cyanoacrylate. Their... Problem 58E: Hot and spicy foods contain molecules that stimulate paindetecting nerve endings. Two such molecules... Problem 59E: One of the first drugs to be approved for use in treatment of acquired immune deficiency syndrome... Problem 60E: The antibiotic thiarubin-A was discovered by studying the feeding habits of wild chimpanzees in... Problem 61E Problem 62E: Sketch the molecular orbital and label its type ( or , bonding or antibonding) that would be formed... Problem 63E Problem 64E: Which of the following are predicted by the molecular orbital model to be stable diatomic species?... Problem 65E Problem 66E Problem 67E Problem 68E: Using the molecular orbital model to describe the bonding in F2+, F2, and F2, predict the bond... Problem 69E Problem 70E: A Lewis structure obeying the octet rule can be drawn for O2 as follows: Use the molecular orbital... Problem 71E: Using the molecular orbital model, write electron configurations for the following diatomic species... Problem 72E: Using the molecular orbital model, write electron configurations for the following diatomic species... Problem 73E: In which of the following diatomic molecules would the bond strength be expected to weaken as an... Problem 74E: In terms of the molecular orbital model, which species in each of the following two pairs will roost... Problem 75E Problem 76E: Show how a hydrogen 1s atomic orbital and a fluorine 2p atomic orbital overlap to form bonding and... Problem 77E: Use Figs. 4-54 and 4-55 to answer the following questions. a. Would the bonding molecular orbital in... Problem 78E: The diatomic molecule OH exists in the gas phase. The bond length and bond energy have been measured... Problem 79E Problem 80E: Describe the bonding in NO+, NO, and NO, using both the localized electron and molecular orbital... Problem 81E: Describe the bonding in the O3 molecule and the NO2 ion, using the localized electron model. How... Problem 82E Problem 83AE Problem 84AE: Vitamin B6 is an organic compound whose deficiency in the human body can cause apathy, irritability,... Problem 85AE: Two structures can be drawn for cyanuric acid: a. Are these two structures the same molecule?... Problem 86AE Problem 87AE: What do each of the following sets of compounds/ions have in common with each other? a. XeCl4, XeCl2... Problem 88AE: Aspartame is an artificial sweetener marketed under the name Nutra-Sweet. A partial Lewis structure... Problem 89AE Problem 90AE: The three most stable oxides of carbon are carbon monoxide (CO), carbon dioxide (CO2), and carbon... Problem 91AE Problem 92AE: Which of the following molecules have net dipole moments? For the molecules that are polar, indicate... Problem 93AE: The strucrure of TeF5 is Draw a complete Lewis structure for TeF5, and explain the distortion from... Problem 94AE: Complete the following resonance structures for POCl3. a. Would you predict the same molecular... Problem 95AE Problem 96AE: Describe the bonding in the first excited state of N2 (the one closest in energy to the ground... Problem 97AE: Using an MO energy-level diagram, would you expect F2 to have a lower or higher first ionization... Problem 98AE: Show how a dxz. atomic orbital and a pz, atomic orbital combine to form a bonding molecular orbital.... Problem 99AE: What type of molecular orbital would result from the in-phase combination of the two dxz atomic... Problem 100AE: Consider three molecules: A, B, and C. Molecule A has a hybridization of sp3 Molecule B has two more... Problem 101CWP Problem 102CWP: Predict the molecular structure, bond angles, and polarity (has a net dipole moment or has no net... Problem 103CWP: Draw the Lewis structures for SO2, PCl3, NNO, COS, and PF3 Which of the compounds are polar? Which... Problem 104CWP: Draw the Lewis structures for TeCl4, ICl5, PCl5, KrCl4, and XeCl2. Which of the compounds exhibit at... Problem 105CWP: A variety of chlorine oxide fluorides and related cations and anions are known. They tend to be... Problem 106CWP: Pelargondin is the molecule responsible for the red color of the geranium flower. It also... Problem 107CWP: Complete a Lewis structure for the compound shown below, then answer the following questions. What... Problem 108CWP Problem 109CWP: Consider the molecular orbital electron configurations for N2, N2+, and N2. For each compound or... Problem 110CWP: Place the species B2+ , B2, and B2 in order of increasing bond length and increasing bond energy. Problem 111CP: The compound NF3 is quite stable, but NCl3 is very unstable (NCl3 was first synthesized in 1811 by... Problem 112CP: Predict the molecular structure for each of the following. (See Exercises 25 and 26.) a. BrFI2 b.... Problem 113CP Problem 114CP: Cholesterol (C27liu;O) has the following structure: In such shorthand structures, each point where... Problem 115CP: Cyanamide (H2NCN), an important industrial chemical, is produced by the following steps: Calcium... Problem 116CP: As compared with CO and O2, CS and S2 are very unstable molecules. Give an explanation based on the... Problem 117CP Problem 118CP: Use the MO model to explain the bonding in BeH2. When constructing the MO energy-level diagram,... Problem 119CP Problem 120CP: Arrange the following from lowest to highest ionization energy: O, O2, O2 , O2+. Explain your... Problem 121CP Problem 122CP Problem 123CP: Carbon monoxide (CO) forms bonds to a variety of metals and metal ions. liS ability to bond to iron... Problem 124CP: The space-filling model for benzoic acid, a food preservative, is shown below. Describe the bonding... Problem 125IP: As the bead engineer of your starship in charge of the warp drive, you notice that the supply of... Problem 126IP: A flask containing gaseous N2 is irradiated with 25-nm light. a. Using the following information,... Problem 127IP: Determine the molecular structure and hybridization of the central atom X in the polyatomic ion XY3+... format_list_bulleted



 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




