
ORGANIC CHEMISTRY-ACCESS
6th Edition
ISBN: 9781260475586
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 53P
For each compound drawn below:
a. Label each
b. Classify each conformation as cis or trans.
c. Translate each structure into a representation with a hexagon for the six-membered ring, and wedges and dashed wedges for groups above and below the ring.
d. Draw the second possible chair condformation for each compound.
[1] 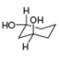 [b]
[b] 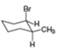 [c]
[c] 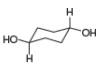
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a structural formula for the major product of the acid-base reaction shown.
H
0
N
+
HCI (1 mole)
CH3
N'
(1 mole)
CH3
You do not have to consider stereochemistry.
●
•
Do not include counter-ions, e.g., Na+, I, in your answer.
. In those cases in which there are two reactants, draw only the product from
989
CH3
344
?
[F
Question 15
What is the major neutral organic product for the following sequence?
1.
POCI₂
pyridine
?
2. OsO4
OH
3. NaHSO
Major Organic Product
✓ OH
OH
'OH
OH
'OH
'CI
URGENT! PLEASE HELP!
Chapter 4 Solutions
ORGANIC CHEMISTRY-ACCESS
Ch. 4.1 - Prob. 1PCh. 4.1 - Problem 4.2 Which of the following is not another...Ch. 4.1 - Problem 4.3 Draw the five constitutional isomers...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.2 - Draw the five constitutional isomers that have...Ch. 4.4 - Problem 4.7 Give the IUPAC name for each...Ch. 4.4 - Give the IUPAC name for each compound. a....Ch. 4.4 - Problem 4.9 Give the structure corresponding to...Ch. 4.4 - Prob. 10P
Ch. 4.5 - Give the IUPAC name for each compound.Ch. 4.5 - Give the structure corresponding to each IUPAC...Ch. 4.8 - Arrange the following compounds in order of...Ch. 4.9 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4.9 - Prob. 15PCh. 4.9 - Prob. 16PCh. 4.10 - Problem 4.17 a. Draw the three staggered and...Ch. 4.10 - Problem 4.18 Rank the following conformations in...Ch. 4.10 - Problem 4.19 Consider rotation around the...Ch. 4.10 - Calculate the destabilization present in each...Ch. 4.12 - Problem 4.21 Classify the ring carbons as up or...Ch. 4.12 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4.13 - Draw a second chair conformation for each...Ch. 4.13 - Problem 4.24 Draw both conformations for and...Ch. 4.13 - Problem 4.25 Draw the structure for each compound...Ch. 4.13 - Prob. 26PCh. 4.14 - Prob. 31PCh. 4.14 - Prob. 32PCh. 4.15 - Prob. 33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 42PCh. 4 - 4.46 Considering rotation around the bond...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 66PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 74PCh. 4 - Prob. 75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
60. The solar system is 25,000 light years from the center of our Milky Way galaxy. One light year is the dista...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forwardCan you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forward
- Part 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 Group of answer choices 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forward
- Calculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0 choices: 0.0269 kJ/mole 2610 kJ/mole 27.6 kJ/mole 0.215 kJ/mole 20.8 kJ/molearrow_forwardCalculate activation energy (Ea) from the following kinetic data: Temp (oC) Time (s) 23.0 180. 32.1 131 40.0 101 51.8 86.0arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License