
(a)
Interpretation: The net ionic equations for the given “molecular” reactions using solubility rules are to be stated. The processes that give a clear visual evidence of an ion exchange process are to be identified.
Concept introduction: When an amount of solute completely dissolves in a solvent, then it is called soluble, and when it is does not completely dissolve it is termed insoluble.
Ionic compounds dissociate into their individual ions in aqueous solution.
Some examples of ionic compounds are sodium chloride, potassium nitrate and potassium chloride.
Some solubility rules for ionic compounds are,
- The alkali metal
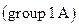 salts are soluble. Examples are Sodium chloride, potassium chloride.
salts are soluble. Examples are Sodium chloride, potassium chloride. - The salts in which nitrates are present are soluble.
- Ammonium salts are soluble.
- Sulfides, carbonates and phosphates are insoluble but sulfides, carbonates and phosphates of the alkali metal and ammonium sulfides, carbonates and phosphates are soluble.
To determine: The balanced net ionic equations for the given “molecular” reactions.
(b)
To determine: The processes that give a clear visual evidence of an ion exchange process.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry: The Science in Context (Fifth Edition)
- In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forwardIf the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forward
- Topic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





