
FUNDAMENTALS OF THERMODYNAMICS
10th Edition
ISBN: 9781119803645
Author: Sonntag
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.62P
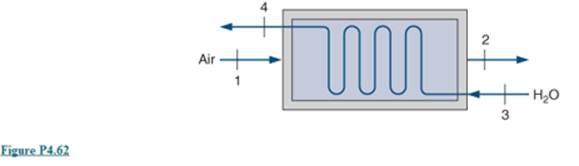
An energy recovery heat exchanger, shown in Fig. P4.62, is used to heat water flow at
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Example-1:
l
D
A uniform rotor of length 0.6 m and diameter 0.4 m is made of steel (density 7810 kg/m³)
is supported by identical short bearings of stiffness 1 MN/m in the horizontal and vertical
directions. If the distance between the bearings is 0.7 m, determine the natural frequencies
and plot whirl speed map.
Solution:
B
find the laplace transform for the
flowing function
2(1-e)
Ans. F(s)=-
S
12)
k
0
Ans. F(s)=
k
s(1+e)
0 a
2a 3a 4a
13)
2+
Ans. F(s)=
1
s(1+e")
3
14) f(t)=1, 0
Find the solution of the following Differential Equations
Using Laplace Transforms
1) 4y+2y=0.
y(0)=2.
y'(0)=0.
2) y+w²y=0,
(0)=A,
y'(0)=B.
3) +2y-8y 0.
y(0)=1.
y'(0)-8.
4)-2-3y=0,
y(0)=1.
y'(0)=7.
5) y-ky'=0,
y(0)=2,
y'(0)=k.
6) y+ky'-2k²y=0,
y(0)=2,
y'(0) = 2k.
7) '+4y=0,
y(0)=2.8
8) y+y=17 sin(21),
y(0)=-1.
9) y-y-6y=0,
y(0)=6,
y'(0)=13.
10) y=0.
y(0)=4,
y' (0)=0.
11) -4y+4y-0,
y(0)=2.1.
y'(0)=3.9
12) y+2y'+2y=0,
y(0)=1,
y'(0)=-3.
13) +7y+12y=21e".
y(0)=3.5.
y'(0)=-10.
14) "+9y=10e".
y(0)=0,
y'(0)=0.
15) +3y+2.25y=91' +64.
y(0)=1.
y'(0) = 31.5
16)
-6y+5y-29 cos(2t).
y(0)=3.2,
y'(0)=6.2
17) y+2y+2y=0,
y(0)=0.
y'(0)=1.
18) y+2y+17y=0,
y(0)=0.
y'(0)=12.
19) y"-4y+5y=0,
y(0)=1,
y'(0)=2.
20) 9y-6y+y=0,
(0)-3,
y'(0)=1.
21) -2y+10y=0,
y(0)=3,
y'(0)=3.
22) 4y-4y+37y=0,
y(0)=3.
y'(0)=1.5
23) 4y-8y+5y=0,
y(0)=0,
y'(0)=1.
24)
++1.25y-0,
y(0)=1,
y'(0)=-0.5
25) y 2 cos(r).
y(0)=2.
y'(0) = 0.
26)
-4y+3y-0,
y(0)=3,
y(0) 7.
27) y+2y+y=e
y(0)=0.
y'(0)=0.
28) y+2y-3y=10sinh(27),
y(0)=0.
y'(0)=4.
29)…
Chapter 4 Solutions
FUNDAMENTALS OF THERMODYNAMICS
Ch. 4 - A temperature difference drives a heat transfer...Ch. 4 - What is the effect can be felt upstream in a flow?Ch. 4 - Prob. 4.3PCh. 4 - Air at 500 kPa is expanded to l00 kPa in two...Ch. 4 - A windmill takes out a fraction of the wind...Ch. 4 - An underwater turbine extracts a fraction of the...Ch. 4 - A liquid water turbine at the bottom of a dam...Ch. 4 - You blow a balloon up with air. What kinds of work...Ch. 4 - Storage tanks of cryogenic liquids (O2,N2,CH4) are...Ch. 4 - A large brewery has a pipe of cross-sectional area...
Ch. 4 - A pool is to be filled with 60m3 water from a...Ch. 4 - Natural gas, CH4 , flowing in a 5cm -diameter pipe...Ch. 4 - A boiler receives a constant flow of 5000kg/h...Ch. 4 - A 0.6m -diameter household fan takes air in at...Ch. 4 - Liquid water at 15°C flows out of nozzle straight...Ch. 4 - A nozzle receives an ideal gas flow with a...Ch. 4 - In a jet engine a flow afar at 1000K,200kPa, and...Ch. 4 - The wind is blowing horizontally at 30m/s in a...Ch. 4 - A meteorite hits the upper atmosphere at 3000m/s ,...Ch. 4 - Carbon dioxide is throttled from 20C,2000kPa to...Ch. 4 - Saturated liquid R-410A at 25°C is throttled to...Ch. 4 - Carbon dioxide used as a natural refrigerant flows...Ch. 4 - Liquid water at 180C,2000kPa is throttled into a...Ch. 4 - Methane at 1MPa,250K is throttled through a valve...Ch. 4 - A steam turbine has an n1et of 3kg/s water at 1200...Ch. 4 - Air at 20m/s,1500K,875kPa with 5kg/s flows into a...Ch. 4 - Solve the previous problem using Table A.7.Ch. 4 - A wind turbine can extract at most a fraction...Ch. 4 - A liquid water turbine receives 2kg/s water at...Ch. 4 - A small high-speed turbine operating on compressed...Ch. 4 - Hoover Dam across the Colorado River dams up Lake...Ch. 4 - What is the specific work one can get from Hoover...Ch. 4 - R-410A in a commercial refrigerator flows into the...Ch. 4 - A compressor brings nitrogen from 100kPa,290K to...Ch. 4 - A refrigerator uses the natural refrigerant carbon...Ch. 4 - A factory generates compressed air from l00kPa,17C...Ch. 4 - A compressor brings R-134a from...Ch. 4 - An exhaust fan in a building should be able to...Ch. 4 - Prob. 4.39PCh. 4 - The air conditioner in a house or a car has a...Ch. 4 - A boiler section boils 3kg/s saturated liquid...Ch. 4 - A superheater takes 3kg/s saturated water vapor in...Ch. 4 - Carbon dioxide enters a steady-state, steady-flow...Ch. 4 - Find the heat transfer in Problem 4.13.Ch. 4 - A chiller cools liquid water for air-conditioning...Ch. 4 - Saturated liquid nitrogen at 600 kPa enters a...Ch. 4 - Prob. 4.47PCh. 4 - Liquid nitrogen at 90K,400kPa flows into a probe...Ch. 4 - Liquid glycol flows around an engine, cooling it...Ch. 4 - An irrigation pump takes water from a river at...Ch. 4 - A pipe from one building to another flows water at...Ch. 4 - A river flowing at 0.5m/s across a 1-m-high and...Ch. 4 - A cutting tool uses a nozzle that generates a...Ch. 4 - An adiabatic steam turbine in a power plant...Ch. 4 - Prob. 4.55PCh. 4 - A steam turbine receives steam from two boilers...Ch. 4 - Prob. 4.57PCh. 4 - Prob. 4.58PCh. 4 - A condenser (heat exchanger) brings 1kg/s water...Ch. 4 - Steam at 500kPa,300C is used to heat cold water at...Ch. 4 - A dual-fluid heat exchanger has 5kg/s water...Ch. 4 - An energy recovery heat exchanger, shown in Fig....Ch. 4 - Do the previous problem if the water is heated to...Ch. 4 - In a co-flowing (same-direction) heat exchanger,...Ch. 4 - An a water counter flowing heat exchanger has one...Ch. 4 - An automotive radiator has glycol at 95°C enter...Ch. 4 - Prob. 4.67PCh. 4 - Two air flows are combined to a single flow. One...Ch. 4 - An open feedwater heater in a power plant heats...Ch. 4 - A de-superheater has a flow of ammonia of 1.5kg/s...Ch. 4 - A mixing chamber with heat transfer receives 2kg/s...Ch. 4 - A geothermal supply of hot water at 500kPa,150C is...Ch. 4 - A flow of 5kg/s water at l00kPa,20C should be...Ch. 4 - A two-stage compressor takes nitrogen ri at...Ch. 4 - The intercooler in the previous problem uses cold...Ch. 4 - Prob. 4.76PCh. 4 - A modern jet engine has a temperature after...Ch. 4 - A proposal is made to use a geothermal supply of...Ch. 4 - Prob. 4.79PCh. 4 - An initially empty canister of volume 0.2m3 is...Ch. 4 - Repeat the previous problem but use the line...Ch. 4 - A tank contains 1m3 air at 100kPa,300K . A pipe...Ch. 4 - A 2.5L tank initially is empty, and we want to...Ch. 4 - An insulated 2m3 tank is to be charged with R-134a...Ch. 4 - Repeat the previous problem if the valve is closed...Ch. 4 - A 3m3 ? cryogenic storage tank contains nitrogen...Ch. 4 - A nitrogen line at 300K,0.5MPa , shown in Fig....Ch. 4 - Prob. 4.88PCh. 4 - A 200L tank (see Fig. P4.89) initially contains...Ch. 4 - A 1-L can of R-410A is at room temperature, 20°C,...Ch. 4 - Steam at 3MPa,400C enters a turbine with a...Ch. 4 - In a glass factory a 2m -wide sheet of glass at...Ch. 4 - Assume a setup similar to that of the previous...Ch. 4 - Three a flows, all at 200 kPa, e connected to the...Ch. 4 - A 1m3,40kg rigid steel tank contains air at 500...Ch. 4 - An insulated spring-loaded piston/cylinder device,...Ch. 4 - A piston/cyl. setup like Fig. 4.96 is such that at...Ch. 4 - A mass-loaded piston/cylinder shown in Fig. P4.98,...Ch. 4 - A flow of 2kg/s of water at 500kPa,20C is heated...Ch. 4 - Refrigerant R-410A at l00psia,60F flows at...Ch. 4 - A pool is to be filled with 2500ft3 water from a...Ch. 4 - Liquid water at 60 F flows out of a nozzle...Ch. 4 - Prob. 4.103EPCh. 4 - Prob. 4.104EPCh. 4 - Nitrogen gas flows into a convergent nozzle at...Ch. 4 - A meteorite hits the upper atmosphere at 10000ft/s...Ch. 4 - Refrigerant R-410A flows out of a cooler at...Ch. 4 - Saturated vapor R-410A at 75 psia is throttled to...Ch. 4 - A wind turbine can exact at most a fraction 16/27...Ch. 4 - A liquid water turbine receives 4Ibm/s water at...Ch. 4 - Prob. 4.111EPCh. 4 - What is the specific work one can get from Hoover...Ch. 4 - A small-speed turbine operating on compressed air...Ch. 4 - R.410A in a commercial refigerator flows into the...Ch. 4 - An exhaust fan in a building should be able to...Ch. 4 - Carbon dioxide gas enters a steady-state,...Ch. 4 - Prob. 4.117EPCh. 4 - Liquid glycol flows around an engine, cooling t as...Ch. 4 - Prob. 4.119EPCh. 4 - Prob. 4.120EPCh. 4 - Do the previous problem if the water is just...Ch. 4 - A dual-fluid heat exchanger has l0Ibm/s water...Ch. 4 - Steam at 80psia,600F is used to heat cold water at...Ch. 4 - Prob. 4.124EPCh. 4 - Two flows of air are both at 30 psia one has...Ch. 4 - A de-superheater has a flow of ammonia of 3Ibm/s...Ch. 4 - A two-stage compressor takes nitrogen n at...Ch. 4 - The intercooler in the previous problem uses cold...Ch. 4 - Prob. 4.129EPCh. 4 - Prob. 4.130EPCh. 4 - A tank contains l0ft3 of air at 15psia,540R . A...Ch. 4 - Prob. 4.132EPCh. 4 - In a glass factory a 6 ft-wide sheet of glass at...Ch. 4 - A mass-loaded piston/cylinder containing air is at...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Auto Controls A union feedback control system has the following open loop transfer function where k>0 is a variable proportional gain i. for K = 1 , derive the exact magnitude and phase expressions of G(jw). ii) for K = 1 , identify the gaincross-over frequency (Wgc) [where IG(jo))| 1] and phase cross-overfrequency [where <G(jw) = - 180]. You can use MATLAB command "margin" to obtain there quantities. iii) Calculate gain margin (in dB) and phase margin (in degrees) ·State whether the closed-loop is stable for K = 1 and briefly justify your answer based on the margin . (Gain marginPhase margin) iv. what happens to the gain margin and Phase margin when you increase the value of K?you You can use for loop in MATLAB to check that.Helpful matlab commands : if, bode, margin, rlocus NO COPIED SOLUTIONSarrow_forwardThe 120 kg wheel has a radius of gyration of 0.7 m. A force P with a magnitude of 50 N is applied at the edge of the wheel as seen in the diagram. The coefficient of static friction is 0.3, and the coefficient of kinetic friction is 0.25. Find the acceleration and angular acceleration of the wheel.arrow_forwardAuto Controls Using MATLAB , find the magnitude and phase plot of the compensators NO COPIED SOLUTIONSarrow_forward
- 4-81 The corner shown in Figure P4-81 is initially uniform at 300°C and then suddenly exposed to a convection environment at 50°C with h 60 W/m². °C. Assume the = 2 solid has the properties of fireclay brick. Examine nodes 1, 2, 3, 4, and 5 and deter- mine the maximum time increment which may be used for a transient numerical calculation. Figure P4-81 1 2 3 4 1 cm 5 6 1 cm 2 cm h, T + 2 cmarrow_forwardAuto Controls A union feedback control system has the following open loop transfer function where k>0 is a variable proportional gain i. for K = 1 , derive the exact magnitude and phase expressions of G(jw). ii) for K = 1 , identify the gaincross-over frequency (Wgc) [where IG(jo))| 1] and phase cross-overfrequency [where <G(jw) = - 180]. You can use MATLAB command "margin" to obtain there quantities. iii) Calculate gain margin (in dB) and phase margin (in degrees) ·State whether the closed-loop is stable for K = 1 and briefly justify your answer based on the margin . (Gain marginPhase margin) iv. what happens to the gain margin and Phase margin when you increase the value of K?you You can use for loop in MATLAB to check that.Helpful matlab commands : if, bode, margin, rlocus NO COPIED SOLUTIONSarrow_forwardAuto Controls Hand sketch the root Focus of the following transfer function How many asymptotes are there ?what are the angles of the asymptotes?Does the system remain stable for all values of K NO COPIED SOLUTIONSarrow_forward
- Please draw the section view of the following problemsarrow_forward7) Please draw the front, top and side view for the following object. Please cross this line outarrow_forwardA 10-kg box is pulled along P,Na rough surface by a force P, as shown in thefigure. The pulling force linearly increaseswith time, while the particle is motionless att = 0s untilit reaches a maximum force of100 Nattimet = 4s. If the ground has staticand kinetic friction coefficients of u, = 0.6 andHU, = 0.4 respectively, determine the velocityof the A 1 0 - kg box is pulled along P , N a rough surface by a force P , as shown in the figure. The pulling force linearly increases with time, while the particle is motionless at t = 0 s untilit reaches a maximum force of 1 0 0 Nattimet = 4 s . If the ground has static and kinetic friction coefficients of u , = 0 . 6 and HU , = 0 . 4 respectively, determine the velocity of the particle att = 4 s .arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license