
Concept explainers
(a)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
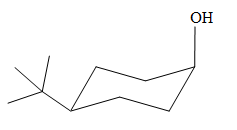
Explanation of Solution
The given compound is
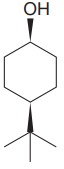
The cyclohexane ring has two substituents attached. One of them is tertiary butyl, and the other is hydroxyl. Both these substituents are on the same side of the ring represented as a wedge line. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring, and it is more stable in the equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond; hence, it must point up in the chair conformation. It is shown below:
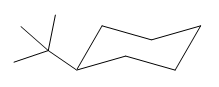
This tertiary butyl group is pointed up, and the hydroxyl group must also point up for them to be cis.

If the chair is flipped, the equatorial tertiary butyl group becomes axial, and the axial hydroxyl group becomes equatorial. This conformation would be less stable since the bulkier group, which is tertiary butyl group, will be at the axial position. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has one substituent in axial position and other in equatorial position.
(b)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
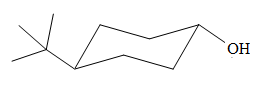
Explanation of Solution
The given compound is
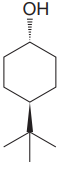
The cyclohexane ring has two substituents attached. One of them is tertiary butyl, and the other is hydroxyl. Both these substituents are on the opposite side of the ring. Thus, they are trans to each other. The tertiary butyl group is the largest substituent on the ring, and it is more stable in the equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond; hence, it must point up in the chair conformation. It is shown below:

This tertiary butyl group is pointed up, and the hydroxyl group must point down for them to be trans.

In the above structure, both the substituents occupy the equatorial position. Thus, this is the most stable conformation for the molecule.
If the chair is flipped, the equatorial tertiary butyl group and hydroxyl group become axial. This conformation would be less stable since the bulkier group, which is tertiary butyl group, will be at the axial position. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has two substituents in the equatorial position.
(c)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
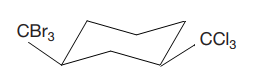
Explanation of Solution
The given compound is
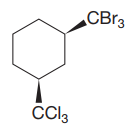
The cyclohexane ring has two substituents attached at C1 and C3 carbon atoms of cyclohexane. One of them is tribromomethane, and the other is trichloromethane. Both these substituents are on the same side of the ring represented as a wedge line. Thus, they are cis to each other. The substituent tribromomethyl is bulkier than trichloromethyl due to the size of bromine atoms. Thus, begin by drawing a chair conformation with tribromomethyl in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:
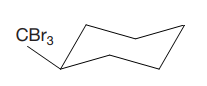
This tribromomethyl group is pointed up, and the trichloromethyl group must also point up for them to be cis.

If the chair is flipped, both the bulky groups will occupy the axial position, which will decrease the stability. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has both the bulky substituents in the quatorial position.
(d)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
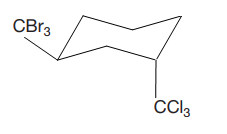
Explanation of Solution
The given compound is
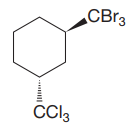
The cyclohexane ring has two substituents attached at C1 and C3 carbon atoms of the cyclohexane. One of them is tribromomethane, and the other is trichloromethane. Both these substituents are on the opposite side of the ring represented as a wedge and a dash line. Thus, they are trans to each other. The substituent tribromomethyl is bulkier than trichloromethyl due to the relative size of bromine. Thus, begin by drawing a chair conformation with tribromomethyl in the equatorial position. It is shown by a wedge bond; hence, it must point up in the chair conformation. It is shown below:

This tribromomethyl group is pointed up, and the trichloromethyl group must point down for them to be trans.

If the chair is flipped, tribromomethyl would occupy the axial position and trichloromethyl gruop will occupy the equatorial position. Tribromomethyl substituent is bulkier than trichloromethyl group. Hence, this is the most stable chair conformation of the given molecule in which the tribromomethyl group is in the equatorial position.
The most stable conformation of the given molecule has the bulkier substituents in the equatorial position.
(e)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is:
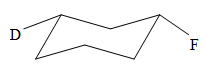
Explanation of Solution
The given compound is
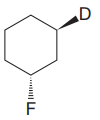
The cyclohexane ring has two substituents attached. One of them is deuterium, and the other is fluorine. Both these substituents are on the opposite side of the ring represented as a wedge and a dash line. Thus, they are trans to each other. The substituent fluorine is bulkier than deuterium. Thus, begin by drawing a chair conformation with fluorine in the equatorial position. It is shown by a dash bond; hence, it must point down in the chair conformation. It is shown below:
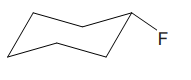
This fluorine group is pointed down, and the deuterium must point down for them to be trans.

If the chair is flipped, both the substituents would occupy the axial position, which will decrease the stability of the chair conformation. Hence, this is the most stable chair conformation of the given molecule in which the two substituents occupy the equatorial position.
The most stable conformation of the given molecule has both the bulky substituents in the equatorial position.
(f)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
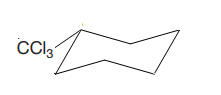
The most stable conformation of the given molecule is
Explanation of Solution
The given compound is
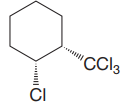
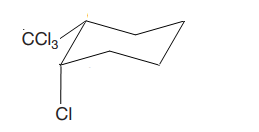
The cyclohexane ring has trichloromethyl group and chlorine at C1 and C2 carbon atoms of cyclohexane. Both these groups are on the same side of the ring. Thus, they are cis to each other. Out of them, trichloromethyl group is bulkier than the chlorine atom, and it is more stable in an equatorial position. Begin by drawing a chair conformation with the trichloromethyl group in the equatorial position. It is shown by a dash bond; hence, it must point down in the chair conformation. It is shown below:
This trichlormethyl group is pointed up, and the methyl group must also point up for them to be cis.

If the chair is flipped, the equatorial trichloromethyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in the equatorial position is more stable. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has bulkier substituent in the equatorial position.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Can I please get help with this.arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
