
Concept explainers
(a)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
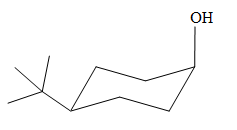
Explanation of Solution
The given compound is
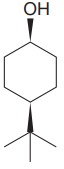
The cyclohexane ring has two substituents attached. One of them is tertiary butyl, and the other is hydroxyl. Both these substituents are on the same side of the ring represented as a wedge line. Thus, they are cis to each other. The tertiary butyl group is the largest substituent on the ring, and it is more stable in the equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond; hence, it must point up in the chair conformation. It is shown below:
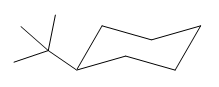
This tertiary butyl group is pointed up, and the hydroxyl group must also point up for them to be cis.

If the chair is flipped, the equatorial tertiary butyl group becomes axial, and the axial hydroxyl group becomes equatorial. This conformation would be less stable since the bulkier group, which is tertiary butyl group, will be at the axial position. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has one substituent in axial position and other in equatorial position.
(b)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
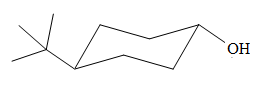
Explanation of Solution
The given compound is
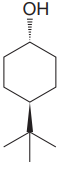
The cyclohexane ring has two substituents attached. One of them is tertiary butyl, and the other is hydroxyl. Both these substituents are on the opposite side of the ring. Thus, they are trans to each other. The tertiary butyl group is the largest substituent on the ring, and it is more stable in the equatorial position. Begin by drawing a chair conformation with a tertiary butyl group in the equatorial position. It is shown by a wedge bond; hence, it must point up in the chair conformation. It is shown below:

This tertiary butyl group is pointed up, and the hydroxyl group must point down for them to be trans.

In the above structure, both the substituents occupy the equatorial position. Thus, this is the most stable conformation for the molecule.
If the chair is flipped, the equatorial tertiary butyl group and hydroxyl group become axial. This conformation would be less stable since the bulkier group, which is tertiary butyl group, will be at the axial position. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has two substituents in the equatorial position.
(c)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
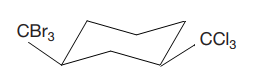
Explanation of Solution
The given compound is
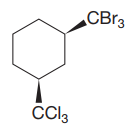
The cyclohexane ring has two substituents attached at C1 and C3 carbon atoms of cyclohexane. One of them is tribromomethane, and the other is trichloromethane. Both these substituents are on the same side of the ring represented as a wedge line. Thus, they are cis to each other. The substituent tribromomethyl is bulkier than trichloromethyl due to the size of bromine atoms. Thus, begin by drawing a chair conformation with tribromomethyl in the equatorial position. It is shown by a wedge bond, hence, it must point up in the chair conformation. It is shown below:
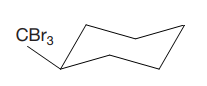
This tribromomethyl group is pointed up, and the trichloromethyl group must also point up for them to be cis.

If the chair is flipped, both the bulky groups will occupy the axial position, which will decrease the stability. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has both the bulky substituents in the quatorial position.
(d)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is
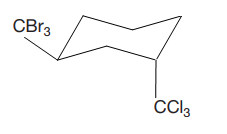
Explanation of Solution
The given compound is
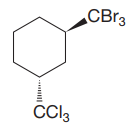
The cyclohexane ring has two substituents attached at C1 and C3 carbon atoms of the cyclohexane. One of them is tribromomethane, and the other is trichloromethane. Both these substituents are on the opposite side of the ring represented as a wedge and a dash line. Thus, they are trans to each other. The substituent tribromomethyl is bulkier than trichloromethyl due to the relative size of bromine. Thus, begin by drawing a chair conformation with tribromomethyl in the equatorial position. It is shown by a wedge bond; hence, it must point up in the chair conformation. It is shown below:

This tribromomethyl group is pointed up, and the trichloromethyl group must point down for them to be trans.

If the chair is flipped, tribromomethyl would occupy the axial position and trichloromethyl gruop will occupy the equatorial position. Tribromomethyl substituent is bulkier than trichloromethyl group. Hence, this is the most stable chair conformation of the given molecule in which the tribromomethyl group is in the equatorial position.
The most stable conformation of the given molecule has the bulkier substituents in the equatorial position.
(e)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
The most stable conformation of the given molecule is:
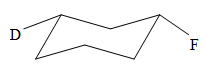
Explanation of Solution
The given compound is
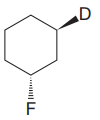
The cyclohexane ring has two substituents attached. One of them is deuterium, and the other is fluorine. Both these substituents are on the opposite side of the ring represented as a wedge and a dash line. Thus, they are trans to each other. The substituent fluorine is bulkier than deuterium. Thus, begin by drawing a chair conformation with fluorine in the equatorial position. It is shown by a dash bond; hence, it must point down in the chair conformation. It is shown below:
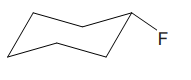
This fluorine group is pointed down, and the deuterium must point down for them to be trans.

If the chair is flipped, both the substituents would occupy the axial position, which will decrease the stability of the chair conformation. Hence, this is the most stable chair conformation of the given molecule in which the two substituents occupy the equatorial position.
The most stable conformation of the given molecule has both the bulky substituents in the equatorial position.
(f)
Interpretation:
The most stable conformation of the given molecule is to be drawn.
Concept introduction:
When the bulkier substituent attached to a cyclohexane ring is in the equatorial position, the chair conformation is more favored. In the disubstituted cyclohexane, the substituents which are on the opposite side of the ring are trans to each other. If there are more than one substituent attached, then the conformation in which maximum substituents are in the equatorial position is favored, and thus it is more stable. Substituents that are trans to each other in one chair conformation remain trans after the chair flip. Substituents that are cis to each other in one chair conformation remain cis after the chair flip.
Answer to Problem 4.46P
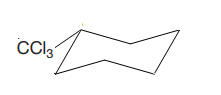
The most stable conformation of the given molecule is
Explanation of Solution
The given compound is
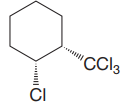
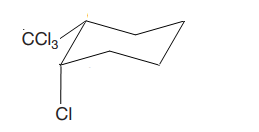
The cyclohexane ring has trichloromethyl group and chlorine at C1 and C2 carbon atoms of cyclohexane. Both these groups are on the same side of the ring. Thus, they are cis to each other. Out of them, trichloromethyl group is bulkier than the chlorine atom, and it is more stable in an equatorial position. Begin by drawing a chair conformation with the trichloromethyl group in the equatorial position. It is shown by a dash bond; hence, it must point down in the chair conformation. It is shown below:
This trichlormethyl group is pointed up, and the methyl group must also point up for them to be cis.

If the chair is flipped, the equatorial trichloromethyl group becomes axial. The chair conformation having the bulkier tertiary butyl group in the equatorial position is more stable. Hence, this is the most stable chair conformation of the given molecule.
The most stable conformation of the given molecule has bulkier substituent in the equatorial position.
Want to see more full solutions like this?
Chapter 4 Solutions
Organic Chemistry: Principles And Mechanisms
- Part 1. Draw monomer units of the following products and draw their reaction mechanism 1) Bakelite like polymer Using: Resorcinol + NaOH + Formalin 2) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerol 3) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardUsing the table of Reactants and Products provided provide the correct letter that corresponds with the Carboxylic acid that is formed in the reaction below. 6 M NaOH Acid-workup WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES A) Pool of Reagents for Part B CI B) OH C) E) CI J) racemic F) K) OH N) OH P) G) OH D) HO H) L) M) HO Q) R) CI Aarrow_forwardIn the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forward
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
