
Inorganic Chemistry
5th Edition
ISBN: 9780321811059
Author: Gary L. Miessler, Paul J. Fischer, Donald A. Tarr
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.28P
For the following molecules, determine the number of IR-active
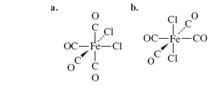
c.
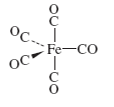
Expert Solution & Answer
Learn your wayIncludes step-by-step video

schedule08:44
Students have asked these similar questions
Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.
Given 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?
3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced?
6 Li + N2 ---> 2 Li3N
Chapter 4 Solutions
Inorganic Chemistry
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Find all the symmetry elements in the following...Ch. 4.2 - Use the procedure described previously to verify...Ch. 4.3 - Prob. 4.4ECh. 4.3 - Verify the transformation matrices for the E and...Ch. 4.3 - Prepare a representation flowchart according to...Ch. 4.4 - Which point groups are possible for chiral...Ch. 4.4 - Write the corresponding 99 transformation matrices...Ch. 4.4 - Using the x, y, and z coordinates for each atom in...Ch. 4.4 - Reduce the following representations to their...
Ch. 4.4 - Prob. 4.11ECh. 4.4 - Analysis of the x, y, and z coordinates of each...Ch. 4.4 - Determine the number of IR-active CO stretching...Ch. 4.4 - Prob. 4.14ECh. 4 - Determine the point groups for a. Ethane...Ch. 4 - Determine the point groups for a. Ethylene b....Ch. 4 - Determine the point groups for a. Acetylene b....Ch. 4 - Determine the point groups for a. Naphthalene b....Ch. 4 - Determine the point groups for a. 1,1’ ...Ch. 4 - Determine the point groups for a. Cyclohexane...Ch. 4 - Determine the point groups for a. A sheet of...Ch. 4 - Determine the point groups for a. A flat oval...Ch. 4 - Determine the point groups for a. A triangular...Ch. 4 - Determine the point groups for the examples of...Ch. 4 - Determine the point groups of the molecules in the...Ch. 4 - Determine the point groups of the molecules and...Ch. 4 - Determine the point groups of the following atomic...Ch. 4 - a. Show that a cube has the same symmetry elements...Ch. 4 - Suppose an octahedron can have either yellow or...Ch. 4 - What point groups are represented by the symbols...Ch. 4 - Prob. 4.17PCh. 4 - Determine the point groups for the following flags...Ch. 4 - Prepare a representation flowchart according to...Ch. 4 - For trans-1,2-dichloroethylene, which has C2h...Ch. 4 - Ethylene has D2h symmetry. a. List all the...Ch. 4 - Using the D2d character table, a. Determine the...Ch. 4 - Reduce the following representations to...Ch. 4 - For D4h symmetry use sketches to show that dxy...Ch. 4 - Prob. 4.25PCh. 4 - XeOF4 has one of the more interesting structures...Ch. 4 - Repeat the procedure from the previous problem,...Ch. 4 - For the following molecules, determine the number...Ch. 4 - Prob. 4.29PCh. 4 - The structure of 1,1,2,2-tetraiododisilane is...Ch. 4 - Both cis and trans isomers of IO2F4 have been...Ch. 4 - White elemental phosphorus consists of tetrahedral...Ch. 4 - Complexes of the general formula Fe(CO)5x( PR3)x...Ch. 4 - Prob. 4.35PCh. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - Prob. 4.38PCh. 4 - Determine the point groups of the following...Ch. 4 - Prob. 4.40PCh. 4 - Determine the point groups of the following: a....Ch. 4 - Use the Internet to search for molecules with the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is an endospore called a resting structure? Of what advantage is an endospore to a bacterial cell?
Microbiology: An Introduction
What is the difference between cellular respiration and external respiration?
Human Physiology: An Integrated Approach (8th Edition)
Based on your answers to Questions 2 and 3, which part of the Atlantic basin appears to have opened first?
Applications and Investigations in Earth Science (9th Edition)
Choose the best answer to each of the following Explain your reasoning. 8.How does the habitable zone around a ...
Cosmic Perspective Fundamentals
1.1 Write a one-sentence definition for each of the following:
a. chemistry
b. chemical
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
MAKE CONNECTIONS The gene that causes sickle-cell disease is present in a higher percentage of residents of su...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
- Explain why the following names of the structures are incorrect. CH2CH3 CH3-C=CH-CH2-CH3 a. 2-ethyl-2-pentene CH3 | CH3-CH-CH2-CH=CH2 b. 2-methyl-4-pentenearrow_forwardDraw the line-angle formula of cis-2,3-dichloro-2-pentene. Then, draw the line-angle formula of trans-2,3-dichloro-2-pentene below. Draw the dash-wedge formula of cis-1,3-dimethylcyclohexane. Then, draw the dash-wedge formula of trans-1,3-dimethylcyclohexane below.arrow_forwardRecord the amounts measured and calculate the percent yield for Part 2 in the table below. Dicyclopentadiene measured in volume Cyclopentadiene measured in grams 0 Measured Calculated Mol Yield Mass (g) or Volume (mL) Mass (g) or Volume (ml) 0.6 2.955 Part 2 Measurements and Results Record the amounts measured and calculate the percent yield for Part 2 in the table below. 0.588 0.0044 2.868 0.0434 N/A Table view List view Measured Calculated Mol $ Yield Melting Point (C) Mass (g) or Volume (ml) Mass (g) or Volume (ml.) Cyclopentadiene 0.1 0.08 0.001189 measured in volume Maleic Anhydride 0.196 N/A cis-norbornene-5,6-endo- dicarboxylic anhydride 0.041 0.0002467 N/A N/A N/A 0.002 N/A N/A 128arrow_forward
- Draw the condensed structural formula and line-angle formula for each: 2,3-dimethylheptane 3-bromo-2-pentanol 3-isopropyl-2-hexene 4-chlorobutanoic acidarrow_forwardRecord the IUPAC names for each of the structures shown below. a) b) c) OH d) OH e)arrow_forwardA solution of 14 g of a nonvolatile, nonelectrolyte compound in 0.10 kg of benzene boils at 81.7°C. If the BP of pure benzene is 80.2°C and the K, of benzene is 2.53°C/m, calculate the molar mass of the unknown compound. AT₁ = Km (14)arrow_forward
- Please help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.arrow_forwardDraw the arrow pushing reaction mechanism. DO NOT ANSWER IF YOU WONT DRAW IT. Do not use chat gpt.arrow_forwardComplete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Photochemistry : Introduction to Basic Theory of Photochemical Process [Part 1]; Author: Dr. Vikrant Palekar;https://www.youtube.com/watch?v=2NDOL11d6no;License: Standard YouTube License, CC-BY
Photochemistry-1; Author: CH-08:ARYABHATT [Mathematics, Physics, Chemistry];https://www.youtube.com/watch?v=DC4J0t1z3e8;License: Standard Youtube License