
Concept explainers
What is the geometry around the central atom in the following molecular models? (There are no “hidden” atoms: all atoms in each model are visible.)
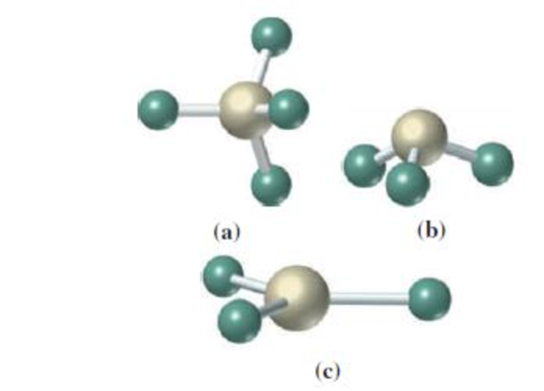
(a)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like a trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is tetrahedral.
Explanation of Solution
In the given molecular model,
Four atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is tetrahedral.
(b)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like a trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is pyramidal.
Explanation of Solution
In the given molecular model,
Three atoms are bonded to the central atom. Three atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is pyramidal.
(c)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is trigonal planar.
Explanation of Solution
In the given molecular model,
Three atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is trigonal planar.
Want to see more full solutions like this?
Chapter 4 Solutions
EP FUND.OF GENERAL,ORG...-MOD.MASTERING
- Biochemistry Please help. Thank you When carbamyl phosphate is joined to L-ornathine, where does the energy for the reaction come from?arrow_forwardBiochemistry Question Please help. Thank you What is the function of glutamate dehydrogenase?arrow_forwardBiochemistry Question Please help. Thank you How and why does a high protein diet affect the enzymes of the urea cycle?arrow_forward
- Biochemistry What is the importance of the glucose-alanine cycle?arrow_forwardBiochemistry Assuming 2.5 molecules of ATP per oxidation of NADH/(H+) and 1.5molecules of ATP per oxidation of FADH2, how many ATP are produced per molecule of pyruvate? Please help. Thank youarrow_forward1. How would you explain the term ‘good food’? 2. How would you define Nutrition? 3. Nutrients are generally categorised into two forms. Discuss.arrow_forward
- Biochemistry Question. Please help solve. Thank you! Based upon knowledge of oxidation of bioorganic compounds and howmuch energy is released during their oxidation, rank the following, from most to least, with respect to how much energy would be produced from each during their oxidation. Explain your placement for each one.arrow_forwardBiochemistry Question.For the metabolism of amino acids what is the first step for theirbreakdown? Why is it necessary for this breakdown product to be transported to the liver? For the catabolism of the carbon backbone of these amino acids, there are 7 entry points into the “standard” metabolic pathways. List these 7 entry points and which amino acids are metabolized to these entry points. Please help. Thank you!arrow_forwardBiochemistry Question. Please help. Thank you. You are studying pyruvate utilization in mammals for ATP production under aerobic conditions and have synthesized pyruvate with Carbon #1 labelled with radioactive C14. After only one complete cycle of the TCA cycle, which of the TCA cycle intermediates would be labeled with C14? Explain your answer. Interestingly, you find C14 being excreted in the urine. How does it get there?arrow_forward
- Biochemistry question. Please help with. Thanks in advance For each of the enzymes listed below, explain what the enzyme does including function, names (or structures) of the substrate and products and the pathway(s) (if applicable) it is/are found in. (a) ATP synthetase (b) succinate dehydrogenase (c) isocitrate lyase (d) acetyl CoA carboxylase (e) isocitrate dehydrogenase (f) malate dehydrogenasearrow_forwardDraw and name each alcohol and classify it as primary, secondary, or tertiary. Explain your answer thoroughly.arrow_forwardDraw the product of each reaction. If there are multiple products, draw only the major product. Explain your answer thoroughly.arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





