
Concept explainers
Use a search engine such as Google to find information about Gordon E. Moore and Moore’s law, the famous law about technological advances that he proposed.
(a) What is Moore’s law? Give a brief description in your own words.
(b) Who is Gordon E. Moore? What was his position at the time he first proposed Moore’s law? What company did he later cofound? With whom did he cofound this company?
(c) In what field did Gordon E. Moore obtain his BS degree? At what university did he receive his BS degree? Where did he obtain his PhD degree? In what field was his PhD degree?
(d) What Nobel Prize-winning physicist gave Gordon E. Moore his first job opportunity?
(e) What was the number of the first microprocessor developed at Moore’s company and how many transistors did it have! When was it introduced!
(f) Read the article by Thomas Friedman on Moore’s law, and watch the video of his interview of Gordon E. Moore.9 In what year in what publication did Moore make his prediction? What was the most important lesson that Moore learned from his law? What stimulated his interest in science and engineering? What does Moore see as the biggest problem in science? How would you describe thestatus of Moore’s Law today!
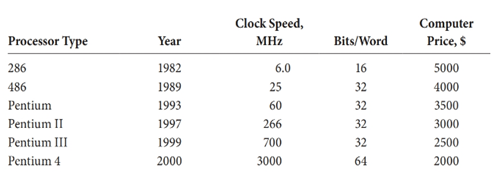
(g) One important benchmark ofcomputational progress is the performance-to-price ratio (PPR) of computers.10 The PPR is the number of bits per word divided by the product of cycle time (1/clockspeed) and price. The original IBM PC (1981) with an 8bit word length, a 4.77 MHz clock, and a pricetag of $5000 came in with a PPR of ~7600. Computers based on other processors available in 2000 are listed in the following table.10 Calculate the PPR of each of these computers. Does Moore’s law hold forthe PPR? How did you come to your conclusion?
Want to see the full answer?
Check out a sample textbook solution
- Don't used hand raiting and don't used Ai solutionarrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception tothe general ionization energy (IE) trend. For the two elements involved, answer the followingquestions. Be sure to cite sources for all physical data that you use.a. (2 pts) Identify the two elements and write their electronic configurations.b. (2 pts) Based on their configurations, propose a reason for the IE trend exception.c. (5 pts) Calculate effective nuclear charges for the last electron in each element and theAllred-Rochow electronegativity values for the two elements. Can any of these valuesexplain the IE trend exception? Explain how (not) – include a description of how IErelates to electronegativity.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- please solve this, and help me know which boxes to check. Thank you so much in advance.arrow_forwardElectronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. Describe how electronegativity is illustrated on the periodic table including trends between groups and periods and significance of atom size.arrow_forwardDefine the term “transition.” How does this definition apply to the transition metals?arrow_forward
- Describe how the properties of the different types of elements (metals, nonmetals, metalloids) differ.arrow_forwardUse a textbook or other valid source to research the physical and chemical properties of each element listed in Data Table 1 using the following as a guideline: Ductile (able to be deformed without losing toughness) and malleable (able to be hammered or pressed permanently out of shape without breaking or cracking) or not ductile or malleable Good, semi, or poor conductors of electricity and heat High or low melting and boiling points Occur or do not occur uncombined/freely in nature High, intermediate, or low reactivity Loses or gains electrons during reactions or is not reactivearrow_forwardProvide the Physical and Chemical Properties of Elements of the following elements listedarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





