
Concept explainers
(a)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but the number of neutrons is different.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
Explanation of Solution
The
The
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
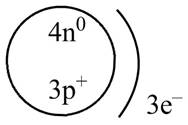
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
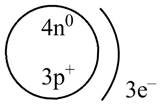
Figure 1
The diagram that represents the arrangement of protons, neutrons, and electrons in
(b)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
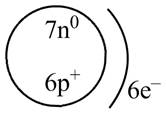
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
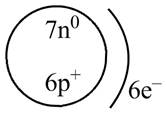
Figure 2
The diagram that represents the arrangement of protons, neutrons, and electrons in
(c)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
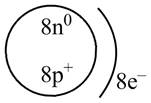
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
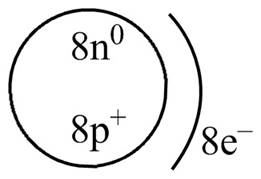
Figure 3
The diagram that represents the arrangement of protons, neutrons, and electrons in
(d)
Interpretation:
The diagram that represents the arrangement of protons, neutrons, and electrons in
Concept introduction:
An atom is made up of three subatomic particles-neutrons, protons, and electrons. Neutron and protons are present in the nucleus of the atom, whereas electrons are revolving outside the nucleus in an atom. Isotopes are those atoms that have the same number of protons but a different number of neutrons.
Answer to Problem 25E
The diagram that represents the arrangement of protons, neutrons, and electrons in
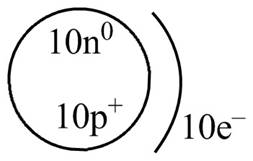
Explanation of Solution
The atomic mass of
The atomic number of
The number of electrons and number of protons in a neutral atom is equal to its atomic number. Hence, the number of protons and number of electrons present in
The atomic mass of the element is represented by the formula as shown below.
Where,
•
•
Rearrange the above equation for the value of
Substitute the value of
One atom of
Therefore, the diagram that represents the arrangement of protons, neutrons, and electrons in
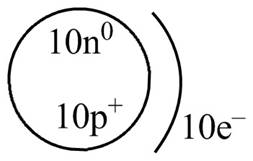
Figure 4
The diagram that represents the arrangement of protons, neutrons, and electrons in
Want to see more full solutions like this?
Chapter 4 Solutions
EBK INTRODUCTORY CHEMISTRY
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





