
Concept explainers
Figure 4.5 Energy inputs and outputs in
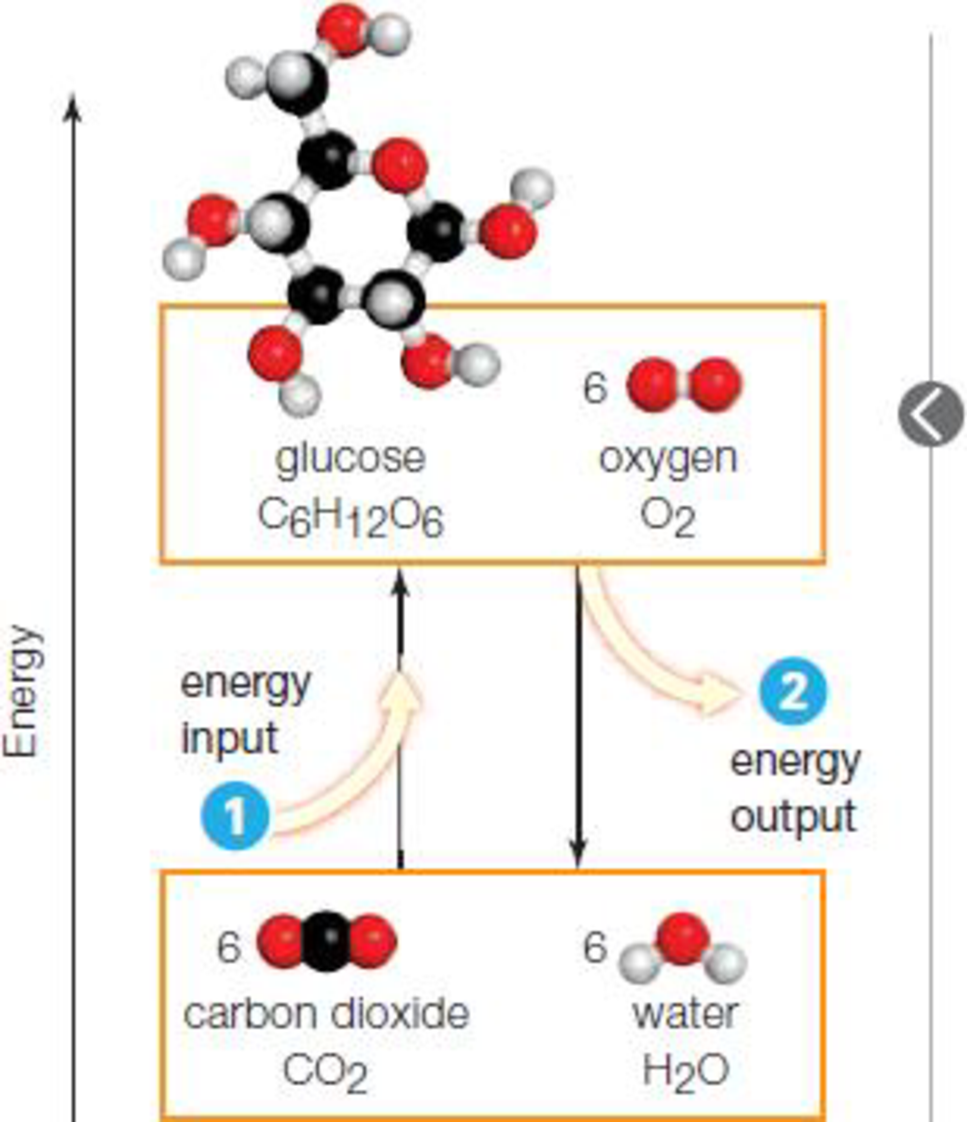
- 1 Some reactions convert molecules with lower energy to molecules with higher energy, so they require a net energy input in order to proceed.
- 2 Other reactions convert molecules with higher energy to molecules with lower energy, so they end with an energy release
Figure It Out: Which law of
To determine: The law of thermodynamics that explains the energy inputs and outputs in chemical reaction.
Introduction: Thermodynamics is defined as the study of heat as well as other forms of energy. In the term thermodynamics, therm indicates heat and dynam indicates power. The first law of thermodynamics states that energy can neither be created nor destroyed. The second law of thermodynamics states that the energy can spread out spontaneously.
Explanation of Solution
The first law of thermodynamics explains the energy inputs and energy outputs in the chemical reactions. The first law of thermodynamics is also called as the law of conservation of energy and it states that the energy can neither be created nor destroyed. The total amount of energy that is present before and after conversion remains the same. If the energy present in reactants is less than the product, then the reaction will proceed only with the net energy input. If the energy of the reactant is higher than the product, the reaction ends with a net energy release.
In the given figure, a lower energy molecule is converted to a higher energy molecule and for the reaction to proceed, it requires net energy input. In the other reaction, higher energy molecule is converted to the lower energy molecule with a net energy release. Thus, the first law of thermodynamics explains the input and output of energy in the given chemical reactions.
Want to see more full solutions like this?
Chapter 4 Solutions
EBK BIOLOGY TODAY AND TOMORROW WITH PHY
- Figure 6.8 Look at each of the processes shown, and decide if it is endergonic or exergonic. In each case, does enthalpy increase or decrease, and does entropy increase or decrease?arrow_forwardFigure 1 Figure 2 Reaction Reaction Abe notices that the temperature of the mixture in figure 1 gets colder. Abe nota que la temperatura de la mezcla en la figura 1 se vuelve más fría. A) Describe the systems (i.e. in the reaction and its surroundings), in which the energy is conserved, represented by the two figures. B) Explain why energy is neither created nor destroyed in these systems. Energy- Energyarrow_forwardWhich of the following is a description of an example of the second law of thermodynamics? Some chemical energy in glucose transforms to chemical energy in ATP. O The kinetic energy of wind turns the blades of a wind turbine. The chemical energy in gasoline is transformed to kinetic energy to drive a car. The mechanical energy of flowing water turns a turbine. Some chemical energy in gasoline is transformed to heat while driving a car.arrow_forward
- Which of the following best describes a thermodynamically favorable (spontaneous) chemical reaction? It has a ΔG that is greater than zero and is endergonic. It has a ΔG that is greater than zero and is exergonic. It has a ΔG that is less than zero and is endergonic. It has a ΔG that is less than zero and is exergonic.arrow_forwardIn a transition state diagram, which of the following are features of the transition state (TS)? There may be more than one correct answer, select all that apply. The change in energy in ground state to the transition state represents the Gibbs Free Energy If the reaction is reversible, the TS will only progress forward to form products The TS occupies a trough The TS is associated with the highest energy The TS occupies the highest peakarrow_forwardthis one represents an endothermic reaction. Things are similar: the flat line on the left (beginning of the reaction) is the total energy possessed by the reactant molecules; once again, kJ stands for energy in kiloJoules, thousands of Joules. The flat line on the right (reaction complete) is the total energy of the products. Since an endothermic reaction has a net absorption of energy (taking this extra energy from the surroundings), the products have higher energy than the reactants. Question: the energy of the reactant molecules is kJ. [to answer, simply identify the correct y-axis coordinate.] 250 200 PE (kJ) 150 100 50 Reaction pathwayarrow_forward
- Refer to Model 10.1 and answer the question that follows What is free energy? What is its symbol?arrow_forwardFigure 6.10 If no activation energy were required to break down sucrose (table sugar), would you be able to store it in a sugar bowl? Gibbs Free Energy EXERGONIC REACTION: &G<0 Reaction is spontaneous Activation energy of uncatalyzed reaction Activation enerGYO ▶ catalyzed reaction Figure 6.10 Activation energy is the energy required for a reaction to proceed, and it is lower if the reaction is catalyzed. This diagram's horizontal axis describes the sequence of events in time.arrow_forwardWhich is not true regarding the 1st Law of Thermodynamics? Energy cannot be destroyed. Energy cannot be created Energy can change form. All are truearrow_forward
- How it is that cells are making larger, more complex molecules, yet they do not defy the second law of thermodynamics? (note: cells are open systems not closed)arrow_forwardDiagrams like the one below are used to represent exothermic reactions. For instance, consider burning charcoal (which almost entirely pure element carbon). A=carbon and B=oxygen, when they react they form C=carbon dioxide. A little chemistry vocab: does substance C in the diagram represent a reactant or the product? Activation Energy A+B Reaction Progression Energyarrow_forwardQuestion:- Q1.Given the following chemical reaction: C6H12O6 + 6 O2 à 6 CO2 + 6 H2O + 36 ATP What process is shown, cellular respiration or photosynthesis? Is this reaction exergonic or endergonic? What are the reactants? Which reactant undergoes oxidation (LEO) to form CO2 What are the products? Which reactant undergoes reduction (GER) to form H2Oarrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning




