
Concept explainers
Figure 4.6 Look at each of the processes shown and decide if it is endergonic or exergonic.

To analyze:
The process shown in the figure is endergonic or exergonic.
Introduction:
The endergonic process is the one which is accompanied by the absorption of energy from the surrounding, while exergonic processes is accompanied by the release of energy into the environment.
Explanation of Solution
Given:
Figure (a) 
Figure (b) 
Figure (c) 
Figure (d) 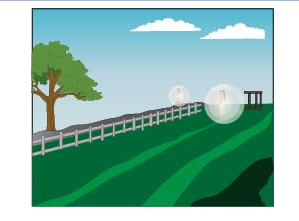
The first figure is of a compost. Decomposition of dead and decaying matter to produce compost or humus is an exergonic process because it is accompanied by the release of heat energy.
The second picture is of a chick coming out from an egg. It is an endergonic process because the process has taken in the energy from the chick.
The third picture is of tea dissolving in water, which is an exergonic process.
The last one is of a ball rolling downwards, in this case, the energy is dissipiated in the environment, which is an exothermic or exergonic process.
Decomposition, dissolving of tea in water and rolling of the ball are exergonic reactions, while the hatching of a chick from an egg is an example of an endergonic reaction.
Want to see more full solutions like this?
Chapter 4 Solutions
Concepts of Biology
Additional Science Textbook Solutions
Biological Science (6th Edition)
Human Anatomy & Physiology (2nd Edition)
Microbiology with Diseases by Body System (5th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Concepts of Genetics (12th Edition)
Biology: Life on Earth (11th Edition)
- Please indentify the unknown organismarrow_forwardPlease indentify the unknown organismarrow_forward5G JA ATTC 3 3 CTIA A1G5 5 GAAT I I3 3 CTIA AA5 Fig. 5-3: The Eco restriction site (left) would be cleaved at the locations indicated by the arrows. However, a SNP in the position shown in gray (right) would prevent cleavage at this site by EcoRI One of the SNPs in B. rapa is found within the Park14 locus and can be detected by RFLP analysis. The CT polymorphism is found in the intron of the Bra013780 gene found on Chromosome 1. The Park14 allele with the "C" in the SNP has two EcoRI sites and thus is cleaved twice by EcoRI If there is a "T" in that SNP, one of the EcoRI sites is altered, so the Park14 allele with the T in the SNP has only one EcoRI site (Fig. 5-3). Park14 allele with SNP(C) Park14 allele with SNPT) 839 EcoRI 1101 EcoRI 839 EcoRI Fig. 5.4: Schematic restriction maps of the two different Park14 alleles (1316 bp long) of B. rapa. Where on these maps is the CT SNP located? 90 The primers used to amplify the DNA at the Park14 locus (see Fig. 5 and Table 3 of Slankster et…arrow_forward
- From your previous experiment, you found that this enhancer activates stripe 2 of eve expression. When you sequence this enhancer you find several binding sites for the gap gene, Giant. To test how Giant interacts with eve, you decide to remove all of the Giant binding sites from the eve enhancer. What results do you expect to see with respect to eve expression?arrow_forwardWhat experiment could you do to see if the maternal gene, bicoid, is sufficient to form anterior fates?arrow_forwardYou’re curious about the effect that gap genes have on the pair-rule gene, evenskipped (eve), so you isolate and sequence each of the eve enhancers. You’re particularly interested in one of the enhancers, which is just upstream of the eve gene. Describe an experimental technique you would use to find out where this particular eve enhancer is active.arrow_forward
- For short answer questions, write your answers on the line provided. To the right is the mRNA codon table to use as needed throughout the exam. First letter U บบบ U CA UUCPhe UUA UCU Phe UCC UUG Leu CUU UAU. G U UAC TV UGCys UAA Stop UGA Stop A UAG Stop UGG Trp Ser UCA UCG CCU] 0 CUC CUA CCC CAC CAU His CGU CGC Leu Pro CCA CAA Gin CGA Arg CUG CCG CAG CGG AUU ACU AAU T AUC lle A 1 ACC Thr AUA ACA AUG Mot ACG AGG Arg GUU GCU GUC GCC G Val Ala GAC Asp GGU GGC GUA GUG GCA GCG GAA GGA Gly Glu GAGJ GGG AACASH AGU Ser AAA1 AAG Lys GAU AGA CAL CALUCAO CAO G Third letter 1. (+7) Use the table below to answer the questions; use the codon table above to assist you. The promoter sequence of DNA is on the LEFT. You do not need to fill in the entire table. Assume we are in the middle of a gene sequence (no need to find a start codon). DNA 1 DNA 2 mRNA tRNA Polypeptide C Val G C. T A C a. On which strand of DNA is the template strand (DNA 1 or 2)?_ b. On which side of the mRNA is the 5' end (left or…arrow_forward3. (6 pts) Fill in the boxes according to the directions on the right. Structure R-C R-COOH OH R-OH i R-CO-R' R R-PO4 R-CH3 C. 0 R' R-O-P-OH 1 OH H R-C-H R-N' I- H H R-NH₂ \H Name Propertiesarrow_forward4. (6 pts) Use the molecule below to answer these questions and identify the side chains and ends. Please use tidy boxes to indicate parts and write the letter labels within that box. a. How many monomer subunits are shown? b. Box a Polar but non-ionizable side chain and label P c. Box a Basic Polar side chain and label BP d. Box the carboxyl group at the end of the polypeptide and label with letter C (C-terminus) H H OHHO H H 0 HHO H-N-CC-N-C-C N-C-C-N-GC-OH I H-C-H CH2 CH2 CH2 H3C-C+H CH2 CH2 OH CH CH₂ C=O OH CH2 NH2arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage LearningCase Studies In Health Information ManagementBiologyISBN:9781337676908Author:SCHNERINGPublisher:Cengage Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning





