
(a)
Interpretation:
The foods in Table
(a)
Explanation of Solution
The Table
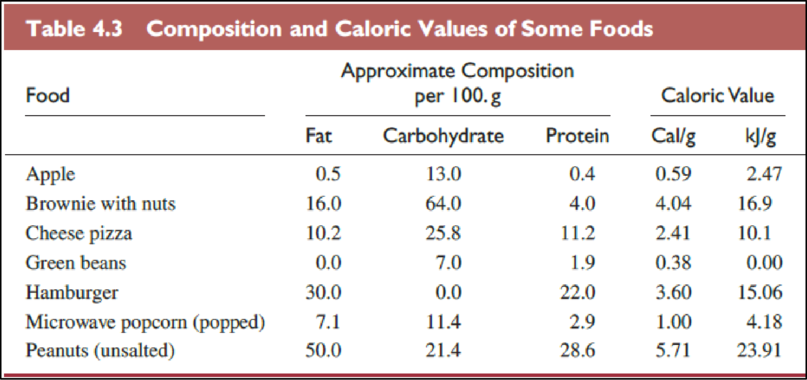
The food items in above table are ranked from low caloric value to high caloric value as follows,
(b)
Interpretation:
The foods in Table
(b)
Explanation of Solution
The Table
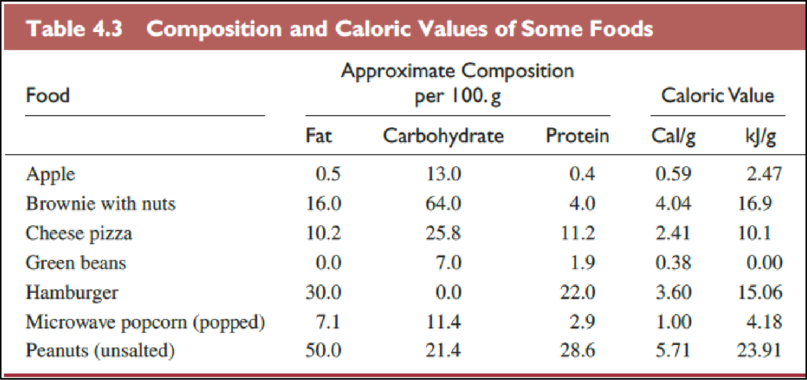
The food items in above table are ranked from low fat content per
(c)
Interpretation:
The foods in Table
(c)
Explanation of Solution
The Table
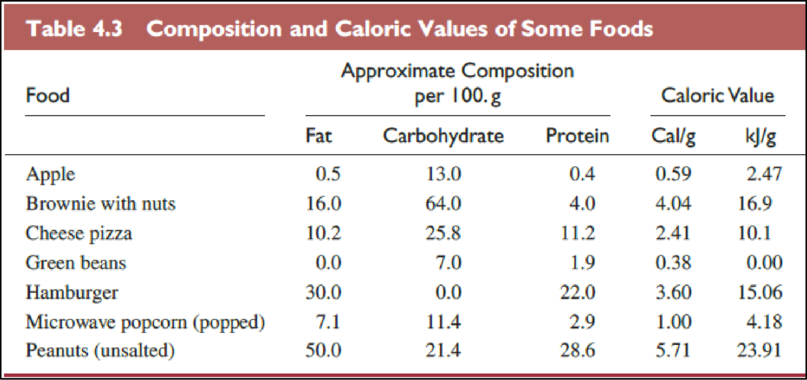
The food items in above table are ranked from most healthful to least one as follows,
Observing sub parts (a) and (b) shows that they matches very well since only 2 items are interchanged. The third list discussed sub part (c) had two high fat foods closer to top of the list, due to fact that protein is important for a healthy diet. Hamburger and peanuts are good protein sources.
Advantage of using caloric value/fat content in food evaluation is that it helps individuals to understand which food helps in weight reduction or weight gain of the individuals. This is achieved by finding alternatives food without consuming more fuel than needed.
Disadvantage of caloric value/fat content in food evaluation is that fats and proteins are important nutritional components of healthy diet. Some amounts of proteins and fats has to be included in daily diet to achieve healthy life style.
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry: The Molecular Science
- One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.arrow_forwardHow does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forward
- Benzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forwardDraw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forward
- pls helparrow_forwardpls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





