
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
9th Edition
ISBN: 9781305922198
Author: John E. McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3.SE, Problem 33AP
Interpretation Introduction
Interpretation:
The organic compound, monobromo derivative C5H11Br is to be drawn and named.
Concept introduction:
C5H11Br is an example of pentane in which one of the hydrogen atom is replaced by the bromine group.
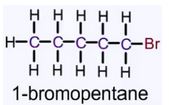
Expert Solution & Answer
Trending nowThis is a popular solution!
Learn your wayIncludes step-by-step video

schedule06:34
Students have asked these similar questions
Using wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has R stereochemical configuration.
NH
H
Br
X
टे
Provide photos of models of the following molecules. (Include a key for identification of the atoms)
1,2-dichloropropane
2,3,3-trimethylhexane
2-bromo-3-methybutane
Please draw the structure in the box that is consistent with all the spectral data and
alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all
the proton NMR peaks. The integrations are computer generated and approximate the number of
equivalent protons. Molecular formula: C13H1802
14
13
12
11
10
11 (ppm)
Structure with assigned H peaks
2.08
3.13
Chapter 3 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
Ch. 3.1 - Prob. 1PCh. 3.1 - Prob. 2PCh. 3.1 - Identify the functional groups in the following...Ch. 3.2 - Draw structures of the five isomers of C6H14.Ch. 3.2 - Propose structures that meet the following...Ch. 3.2 - Prob. 6PCh. 3.3 - Draw the eight 5-carbon alkyl groups (pentyl...Ch. 3.3 - Identify the carbon atoms in the following...Ch. 3.3 - Prob. 9PCh. 3.3 - Prob. 10P
Ch. 3.4 - Give IUPAC names for the following compounds:Ch. 3.4 - Prob. 12PCh. 3.4 - Name the eight 5-carbon alkyl groups you drew in...Ch. 3.4 - Give the IUPAC name for the following hydrocarbon,...Ch. 3.7 - Make a graph of potential energy versus angle of...Ch. 3.7 - Sight along the C2-C1 bond of 2-methylpropane...Ch. 3.7 - Sight along the C2-C3 bond of 2,3-dimethylbutane,...Ch. 3.7 - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 19VCCh. 3.SE - Prob. 20VCCh. 3.SE - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 22APCh. 3.SE - Prob. 23APCh. 3.SE - Propose structures for the following: (a) A...Ch. 3.SE - Prob. 25APCh. 3.SE - Draw the structures of the following molecules:...Ch. 3.SE - Draw structures that meet the following...Ch. 3.SE - Prob. 28APCh. 3.SE - In each of the following sets, which structures...Ch. 3.SE - There are seven constitutional isomers with the...Ch. 3.SE - Prob. 31APCh. 3.SE - Draw compounds that contain the following: (a) A...Ch. 3.SE - Prob. 33APCh. 3.SE - Draw and name all monochloro derivatives of...Ch. 3.SE - Draw structures for the following: (a)...Ch. 3.SE - Prob. 36APCh. 3.SE - Draw a compound that: (a) Has nine primary...Ch. 3.SE - Give IUPAC names for the following compounds:Ch. 3.SE - Name the five isomers of C6H14.Ch. 3.SE - Explain why each of the following names is...Ch. 3.SE - Prob. 41APCh. 3.SE - Consider 2-methylbutane (isopentane). Sighting...Ch. 3.SE - What are the relative energies of the three...Ch. 3.SE - Construct a qualitative potential-energy diagram...Ch. 3.SE - Prob. 45APCh. 3.SE - Draw the most stable conformation of pentane,...Ch. 3.SE - Draw the most stable conformation of...Ch. 3.SE - Prob. 48APCh. 3.SE - Prob. 49APCh. 3.SE - Formaldehyde, H2C=O, is known to all biologists...Ch. 3.SE - Prob. 51APCh. 3.SE - Increased substitution around a bond leads to...Ch. 3.SE - Prob. 53APCh. 3.SE - In the next chapter we'll look at...Ch. 3.SE - We’ll see in the next chapter that there are two...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFirefly luciferin exhibits three rings. Identify which of the rings are aromatic. Identify which lone pairs are involved in establishing aromaticity. The lone pairs are labeled A-D below.arrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 10.0 mL of the base solution, what is the pH of the resulting solution?arrow_forward
- Given a complex reaction with rate equation v = k1[A] + k2[A]2, what is the overall reaction order?arrow_forwardPlease draw the structure in the box that is consistent with all the spectral data and alphabetically label all of the equivalent protons in the structure (Ha, Hb, Hc....) in order to assign all the proton NMR peaks. The integrations are computer generated and approximate the number of equivalent protons. Molecular formula: C13H1802 14 13 12 11 10 11 (ppm) Structure with assigned H peaks 2.08 3.13arrow_forwardCHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the steady-state approximation method. Explain what it consists of.arrow_forward
- CHEMICAL KINETICS. One of the approximation methods for solving the rate equation is the limiting or determining step approximation method. Explain what it consists of.arrow_forwardCHEMICAL KINETICS. Indicate the approximation methods for solving the rate equation.arrow_forwardTRANSMITTANCE เบบ Please identify the one structure below that is consistent with the 'H NMR and IR spectra shown and draw its complete structure in the box below with the protons alphabetically labeled as shown in the NMR spectrum and label the IR bands, including sp³C-H and sp2C-H stretch, indicated by the arrows. D 4000 OH LOH H₂C CH3 OH H₂C OCH3 CH3 OH 3000 2000 1500 HAVENUMBERI-11 1000 LOCH3 Draw your structure below and label its equivalent protons according to the peak labeling that is used in the NMR spectrum in order to assign the peaks. Integrals indicate number of equivalent protons. Splitting patterns are: s=singlet, d=doublet, m-multiplet 8 3Hb s m 1Hd s 3Hf m 2Hcd 2Had 1He 鄙视 m 7 7 6 5 4 3 22 500 T 1 0arrow_forward
- Relative Transmittance 0.995 0.99 0.985 0.98 Please draw the structure that is consistent with all the spectral data below in the box and alphabetically label the equivalent protons in the structure (Ha, Hb, Hc ....) in order to assign all the proton NMR peaks. Label the absorption bands in the IR spectrum indicated by the arrows. INFRARED SPECTRUM 1 0.975 3000 2000 Wavenumber (cm-1) 1000 Structure with assigned H peaks 1 3 180 160 140 120 100 f1 (ppm) 80 60 40 20 0 C-13 NMR note that there are 4 peaks between 120-140ppm Integral values equal the number of equivalent protons 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 2.0 1.0 0.0 fl (ppm)arrow_forwardCalculate the pH of 0.0025 M phenol.arrow_forwardIn the following reaction, the OH- acts as which of these? NO2-(aq) + H2O(l) ⇌ OH-(aq) + HNO2(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,