
Concept explainers
Carbon dioxide gas enters a pipe at 3 MPa and 500 K at a rate of 2 kg/s. CO2 is cooled at constant pressure as it flows in the pipe, and the temperature of the CO2 drops to 450 K at the exit. Determine the volume flow rate and the density of carbon dioxide at the inlet and the volume flow rate at the exit of the pipe using (a) the ideal-gas equation and (b) the generalized compressibility chart. Also, determine (c) the error involved in the first case.
FIGURE P3–89
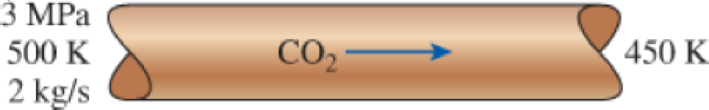
(a)
The volume flow rate, density of carbon dioxide at the inlet, and the volume flow rate at the exit of the pipe using the ideal gas equation of state.
Answer to Problem 89P
The volume flow rate, density of carbon dioxide at the inlet, and the volume flow rate at the exit of the pipe using the ideal gas equation of state are
Explanation of Solution
Refer to Table A-1, obtain the gas constant, critical pressure, and the critical temperature of carbon dioxide.
Write the equation of volume flow rate at the inlet of the pipe.
Here, inlet temperature and inlet pressure are
Calculate the density at the inlet of pipe.
Calculate the equation of volume flow rate at the outlet of the pipe.
Here, outlet temperature and outlet pressure are
Conclusion:
Substitute
Substitute
Substitute
Thus, the volume flow rate, density of carbon dioxide at the inlet, and the volume flow rate at the exit of the pipe using the ideal gas equation of state are
(b)
The volume flow rate, density of carbon dioxide at the inlet, and the volume flow rate at the exit of the pipe using the generalized compressibility chart.
Answer to Problem 89P
The volume flow rate, density of carbon dioxide at the inlet, and the volume flow rate at the exit of the pipe using the generalized compressibility chart are
Explanation of Solution
Calculate the equation of reduced pressure at the inlet of the pipe.
Here, the critical pressure is
Calculate the equation of reduced temperature at the inlet of the pipe.
Here, the critical temperature is
Calculate the equation of reduced pressure at the outlet of the pipe.
Calculate the equation of reduced temperature at the outlet of the pipe.
Write the equation of volume flow rate at the inlet of the pipe.
Here, compressibility factor at the inlet of pipe is
Calculate the density at the inlet of pipe.
Calculate the equation of volume flow rate at the outlet of the pipe.
Here, compressibility factor at the outlet of pipe is
Conclusion:
Substitute 3 MPa for
Substitute 500 K for
Substitute 3 MPa for
Substitute 450 K for
Refer to Figure 3-48, obtain the compressibility factor at inlet state
Refer to Figure 3-48, obtain the compressibility factor at outlet state
Substitute 0.9791 for
Substitute 0.9791 for
Substitute 0.9656 for
Thus, the volume flow rate, density of carbon dioxide at the inlet, and the volume flow rate at the exit of the pipe using the generalized compressibility chart are
(c)
The error involved in the first case.
Answer to Problem 89P
The error involved in the first case are
Explanation of Solution
Calculate the percentage of error involved in the first case of volume flow rate at the inlet condition.
Here, calculated volume flow rate at inlet state from EOS is
Calculate the percentage of error involved in the first case of density at the inlet condition.
Here, calculated density at inlet state from EOS is
Calculate the percentage of error involved in the first case of volume flow rate at the outlet condition.
Here, calculated volume flow rate at outlet state from EOS is
Conclusion:
Substitute
Substitute
Substitute
Thus, the error involved in the first case are
Want to see more full solutions like this?
Chapter 3 Solutions
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
- The primary material used in the production of glass products is silica sand. True or Falsearrow_forwardWhich one of the following is the most common polymer type in fiber-reinforced polymer composites? thermosets thermoplastics elastomers none of the abovearrow_forwardA pattern for a product is larger than the actual finished part. True or Falsearrow_forward
- Two forces are applied as shown to a hook support. The magnitude of P is 38 N. 50 N 25° DG a 터 Using trigonometry, determine the required angle a such that the resultant R of the two forces applied to the support will be horizontal. The value of a isarrow_forwardNo chatgpt pls will upvotearrow_forward101 the three shafts if the diameter ratio is 2 (D/d = 2)? Ans. na, tension = 1.21, na, bending = 1.19, na, torsion = 1.17. 6.32 A material with a yield strength of S₁ = 350 MPa is subjected to the stress state shown in Sketch c. What is the factor of safety based on the maximum shear stress and distortion energy theories? Ans. For MSST, n, = 11.67. 50 MPa 85 MPa 20 MPa 70 MPa Sketch c, for Problems 6.32 and 6.33arrow_forward
- Can you draw the left view of the first orthographic projectionarrow_forwardImportant: I've posted this question twice and received incorrect answers. I've clearly stated that I don't require AI-generated working out. I need a genuine, expert-written solution with proper working. If you can't provide that, refer this question to someone who can please!. Note: Please provide a clear, step-by-step handwritten solution (no AI involvement). I require an expert-level answer and will assess it based on quality and accuracy with that I'll give it a thumbs up or down!. Hence, refer to the provided image for clarity. Double-check everything for correctness before submitting. Thank you!arrow_forwardNote: Please provide a clear, step-by-step simplified handwritten working out (no explanations!), ensuring it is done without any AI involvement. I require an expert-level answer, and I will assess and rate based on the quality and accuracy of your work and refer to the provided image for more clarity. Make sure to double-check everything for correctness before submitting appreciate your time and effort!. Question:arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





