
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
9th Edition
ISBN: 9781260048667
Author: Yunus A. Cengel Dr.; Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3.8, Problem 27P
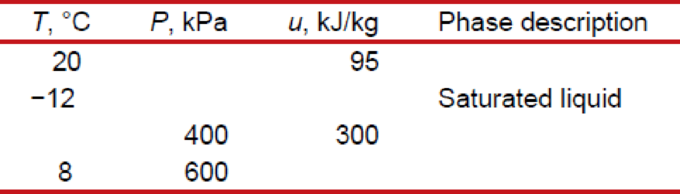
Complete this table for refrigerant-134a:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
This is a tilt and rotation question. Here are notes attached for reference.
I need help with a MATLAB code. For question b.6 I have the MATLAB code shown below. How do I edit the code to answer question b.7. Please make sure the plots are reasonable.
clc;
clear all;
% Constants
mu = 398600; % Earth gravitational parameter, km^3/s^2
% Initial chief and deputy positions and velocities in ECI frame
% Assume circular orbits in equatorial plane for simplicity
a_c = 10000; % km
a_d = 11500; % km
r_c0 = [a_c; 0; 0];
v_c0 = [0; sqrt(mu/a_c); 0];
r_d0 = [a_d; 0; 0];
v_d0 = [0; sqrt(mu/a_d); 0];
% Initial relative state
delta_r0 = r_d0 - r_c0;
delta_v0 = v_d0 - v_c0;
x0 = [delta_r0; delta_v0]; % 6x1 initial relative state
% Time span
tspan = [0 3600]; % 1 hour in seconds
% Damping cases
cases = struct( ...
'name', {'Critically damped', 'Under-damped', 'Over-damped'}, ...
'Kr', {eye(3)*2.5e-3, eye(3)*0.001, eye(3)*0.01}, ...
'P', {eye(3)*0.01, eye(3)*0.0006, eye(3)*0.02} ...
);
% Simulate each case
for i = 1:length(cases)
Kr = cases(i).Kr;
P =…
Just do Questions 7, 9, 11. Here are notes attached for reference.
Chapter 3 Solutions
Thermodynamics: An Engineering Approach ( 9th International Edition ) ISBN:9781260092684
Ch. 3.8 - A propane tank is filled with a mixture of liquid...Ch. 3.8 - Is iced water a pure substance? Why?Ch. 3.8 - What is the difference between saturated vapor and...Ch. 3.8 - What is the difference between saturated liquid...Ch. 3.8 - If the pressure of a substance is increased during...Ch. 3.8 - Is it true that water boils at higher temperature...Ch. 3.8 - What is the difference between the critical point...Ch. 3.8 - A househusband is cooking beef stew for his family...Ch. 3.8 - How does a boiling process at supercritical...Ch. 3.8 - What is quality? Does it have any meaning in the...
Ch. 3.8 - Does the amount of heat absorbed as 1 kg of...Ch. 3.8 - Does the reference point selected for the...Ch. 3.8 - What is the physical significance of hfg? Can it...Ch. 3.8 - Does hfg change with pressure? How?Ch. 3.8 - Is it true that it takes more energy to vaporize 1...Ch. 3.8 - Which process requires more energy: completely...Ch. 3.8 - In what kind of pot will a given volume of water...Ch. 3.8 - It is well known that warm air in a cooler...Ch. 3.8 - In the absence of compressed liquid tables, how is...Ch. 3.8 - A perfectly fitting pot and its lid often stick...Ch. 3.8 - Complete this table for H2O:Ch. 3.8 - Complete this table for H2O:Ch. 3.8 - Complete this table for H2O:Ch. 3.8 - Complete this table for H2O:Ch. 3.8 - Complete this table for refrigerant-134a:Ch. 3.8 - Complete this table for refrigerant-134a:Ch. 3.8 - A 1.8-m3 rigid tank contains steam at 220C....Ch. 3.8 - One pound-mass of water fills a container whose...Ch. 3.8 - A pistoncylinder device contains 0.85 kg of...Ch. 3.8 - 10 kg of R-134a fill a 1.115-m3 rigid container at...Ch. 3.8 - What is the specific internal energy of water at...Ch. 3.8 - What is the specific volume of water at 5 MPa and...Ch. 3.8 - What is the specific volume of R-134a at 20C and...Ch. 3.8 - Refrigerant-134a at 200 kPa and 25C flows through...Ch. 3.8 - One kilogram of R-134a fills a 0.14-m3 weighted...Ch. 3.8 - One kilogram of water vapor at 200 kPa fills the...Ch. 3.8 - The temperature in a pressure cooker during...Ch. 3.8 - How much error would one expect in determining the...Ch. 3.8 - Water is to be boiled at sea level in a...Ch. 3.8 - Repeat Prob. 340 for a location at an elevation of...Ch. 3.8 - 10 kg of R-134a at 300 kPa fills a rigid container...Ch. 3.8 - 100 kg of R-134a at 200 kPa are contained in a...Ch. 3.8 - Water initially at 200 kPa and 300C is contained...Ch. 3.8 - Saturated steam coming off the turbine of a steam...Ch. 3.8 - A person cooks a meal in a 30-cm-diameter pot that...Ch. 3.8 - Water is boiled at 1 atm pressure in a...Ch. 3.8 - Repeat Prob. 347 for a location at 2000-m...Ch. 3.8 - Prob. 49PCh. 3.8 - A rigid tank with a volume of 1.8 m3 contains 40...Ch. 3.8 - A pistoncylinder device contains 0.005 m3 of...Ch. 3.8 - A 5-ft3 rigid tank contains a saturated mixture of...Ch. 3.8 - Superheated water vapor at 180 psia and 500F is...Ch. 3.8 - One kilogram of water fills a 150-L rigid...Ch. 3.8 - 10 kg of R-134a fill a 0.7-m3 weighted...Ch. 3.8 - A pistoncylinder device contains 0.6 kg of steam...Ch. 3.8 - A pistoncylinder device initially contains 1.4 kg...Ch. 3.8 - Water is being heated in a vertical pistoncylinder...Ch. 3.8 - A rigid tank initially contains 1.4 kg saturated...Ch. 3.8 - A pistoncylinder device initially contains 50 L of...Ch. 3.8 - The spring-loaded pistoncylinder device shown in...Ch. 3.8 - A pistoncylinder device initially contains steam...Ch. 3.8 - Under what conditions is the ideal-gas assumption...Ch. 3.8 - What is the difference between mass and molar...Ch. 3.8 - Propane and methane are commonly used for heating...Ch. 3.8 - What is the specific volume of oxygen at 25 psia...Ch. 3.8 - A 100-L container is filled with 1 kg of air at a...Ch. 3.8 - A mass of 1 lbm of argon is maintained at 200 psia...Ch. 3.8 - A 400-L rigid tank contains 5 kg of air at 25C....Ch. 3.8 - The pressure gage on a 2.5-m3 oxygen tank reads...Ch. 3.8 - A spherical balloon with a diameter of 9 m is...Ch. 3.8 - Reconsider Prob. 373. Using appropriate software,...Ch. 3.8 - A 1-m3 tank containing air at 10C and 350 kPa is...Ch. 3.8 - A mass of 10 g of oxygen fill a weighted...Ch. 3.8 - A mass of 0.1 kg of helium fills a 0.2 m3 rigid...Ch. 3.8 - A rigid tank whose volume is unknown is divided...Ch. 3.8 - A rigid tank contains 20 lbm of air at 20 psia and...Ch. 3.8 - In an informative article in a magazine it is...Ch. 3.8 - What is the physical significance of the...Ch. 3.8 - Determine the specific volume of refrigerant-134a...Ch. 3.8 - Refrigerant-134a at 400 psia has a specific volume...Ch. 3.8 - Determine the specific volume of superheated water...Ch. 3.8 - Determine the specific volume of superheated water...Ch. 3.8 - Determine the specific volume of nitrogen gas at...Ch. 3.8 - Prob. 88PCh. 3.8 - Carbon dioxide gas enters a pipe at 3 MPa and 500...Ch. 3.8 - Prob. 90PCh. 3.8 - A 0.016773-m3 tank contains 1 kg of...Ch. 3.8 - Prob. 92PCh. 3.8 - What is the percentage of error involved in...Ch. 3.8 - What is the physical significance of the two...Ch. 3.8 - Refrigerant-134a at 400 psia has a specific volume...Ch. 3.8 - A 3.27-m3 tank contains 100 kg of nitrogen at 175...Ch. 3.8 - Nitrogen at 150 K has a specific volume of...Ch. 3.8 - A 1-m3 tank contains 2.841 kg of steam at 0.6 MPa....Ch. 3.8 - Prob. 103PCh. 3.8 - Prob. 104PCh. 3.8 - On a certain day, the temperature and relative...Ch. 3.8 - Prob. 106PCh. 3.8 - Consider two rooms that are identical except that...Ch. 3.8 - A thermos bottle is half-filled with water and is...Ch. 3.8 - Complete the blank cells in the following table of...Ch. 3.8 - Complete the blank cells in the following table of...Ch. 3.8 - Prob. 111RPCh. 3.8 - Prob. 112RPCh. 3.8 - The gage pressure of an automobile tire is...Ch. 3.8 - A tank contains argon at 600C and 200 kPa gage....Ch. 3.8 - The combustion in a gasoline engine may be...Ch. 3.8 - Prob. 116RPCh. 3.8 - Prob. 117RPCh. 3.8 - A rigid tank with a volume of 0.117 m3 contains 1...Ch. 3.8 - A 9-m3 tank contains nitrogen at 17C and 600 kPa....Ch. 3.8 - A 10-kg mass of superheated refrigerant-134a at...Ch. 3.8 - A 4-L rigid tank contains 2 kg of saturated...Ch. 3.8 - Prob. 123RPCh. 3.8 - A tank whose volume is unknown is divided into two...Ch. 3.8 - Prob. 125RPCh. 3.8 - A tank contains helium at 37C and 140 kPa gage....Ch. 3.8 - Prob. 127RPCh. 3.8 - On the property diagrams indicated below, sketch...Ch. 3.8 - Ethane at 10 MPa and 100C is heated at constant...Ch. 3.8 - Steam at 400C has a specific volume of 0.02 m3/kg....Ch. 3.8 - Consider an 18-m-diameter hot-air balloon that,...Ch. 3.8 - Prob. 135FEPCh. 3.8 - A 3-m3 rigid vessel contains steam at 2 MPa and...Ch. 3.8 - Prob. 137FEPCh. 3.8 - Water is boiled at 1 atm pressure in a coffeemaker...Ch. 3.8 - Prob. 139FEPCh. 3.8 - Water is boiled in a pan on a stove at sea level....Ch. 3.8 - A rigid tank contains 2 kg of an ideal gas at 4...Ch. 3.8 - The pressure of an automobile tire is measured to...Ch. 3.8 - Consider a sealed can that is filled with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- This is a tilt and rotation question. Here are notes attached for reference.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A spring-loaded piston-cylinder device contains 1.5 kg of carbon dioxide. This system is heated from 200kPa and 25◦C to 1200 kPa and 300◦C. Determine the total heat transfer to and work produced by this system.arrow_forwardCan you help with a code in MATLAB?arrow_forward
- I need help writing a code in MATLAB. Please help me with question b.6arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes 1.5-kg of water that is initially at 90◦C with a quality of 5 percent occupies a spring-loaded piston-cylinder device. This device is now heated until the pressure rises to 900 kPa and the temperature is 280◦C. Determinethe total work produced during this process, in kJ.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes Stainless steel ball bearings (ρ = 8085 kg/m3 and cp = 0.480 kJ/(kg◦C)) having a diameter of 1.5 cm areto be quenched in water at a rate of 900 per minute. The balls leave the oven at a uniform temperature of1000◦C and are exposed to air at 25◦C for a while before they are dropped into the water. If the temperatureof the balls drops to 900◦C prior to quenching, determine the rate of heat transfer from the balls to the air.arrow_forward
- Thermodynamics: Mass and Energy Analysis Of Control Volumes A 12-ft3 tank contains oxygen at 15 psia and 80◦F. A paddle wheel within the tank is rotated until thepressure inside rises to 20 psia. During the process 25 Btu of heat is lost to the surroundings. Determine thepaddle wheel work done. Neglect the energy stored in the paddle wheel.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A frictionless piston-cylinder device contains 4.5 kg of nitrogen at 110 kPa and 200 K. Nitrogen is nowcompressed slowly according to the relation PV1.5 = constant until it reaches a final temperature of 360 K.Calculate the work input during the process, in kJ.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes An insulated piston-cylinder device contains 4 L of saturated liquid water at a constant pressure of 200 kPa.Water is stirred by a paddle wheel while a current of 8 A flows for 50 min through a resistor placed in thewater. If one-half of the liquid is evaporated during this constant-pressure process and the paddle-wheelwork amounts to 300 kJ, determine the voltage of the source. Also, show the process on a P–v diagram withrespect to the saturation lines.arrow_forward
- Thermodynamics: Mass and Energy Analysis Of Control Volumes The state of liquid water is changed from 55 psia and 45◦F to 2000 psia and 120◦F. Determine the change inthe internal energy and enthalpy of water on the basis of the (a) compressed liquid tables, (b) incompressiblesubstance approximation and property tables, and (c) specific-heat model.arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes What is the change in enthalpy, in kJ/kg, of oxygen as its temperature changes from 150 to 250◦C? Is thereany difference if the temperature change were from −50 to 100◦C? Does the pressure at the beginning andend of this process have any effect on the enthalpy change?arrow_forwardThermodynamics: Mass and Energy Analysis Of Control Volumes A 50-L electrical radiator containing heating oil is placed in a 50-m3 room. Both the room and the oil in theradiator are initially at 5◦C. The radiator with a rating of 3 kW is now turned on. At the same time, heatis lost from the room at an average rate of 0.3 kJ/s. After some time, the average temperature is measuredto be 20◦C for the air in the room, and 60◦C for the oil in the radiator. Taking the density and the specificheat of the oil to be 950 kg/m3 and 2.2 kJ/(kg◦C), respectively, determine how long the heater is kept on.Assume the room is well-sealed so that there are no air leaks.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning

Refrigeration and Air Conditioning Technology (Mi...
Mechanical Engineering
ISBN:9781305578296
Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill Johnson
Publisher:Cengage Learning
The Refrigeration Cycle Explained - The Four Major Components; Author: HVAC Know It All;https://www.youtube.com/watch?v=zfciSvOZDUY;License: Standard YouTube License, CC-BY