
Concept explainers
Complete the blank cells in the following table of properties of refrigerant-134a. In the last column, describe the condition of refrigerant-134a as compressed liquid, saturated mixture, superheated vapor, or insufficient information, and, if applicable, give the quality.
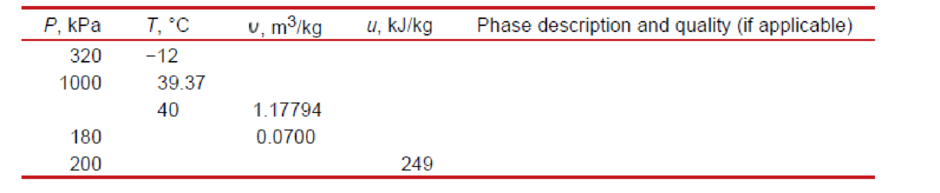
The following table for refrigerant-134a which are blank.
| P, kPa | u, kJ/kg | x, quality | Phase description | ||
| 320 | -12 | ||||
| 1000 | 39.37 | ||||
| 40 | 1.17794 | ||||
| 180 | 0.0700 | ||||
| 200 | 249 |
Explanation of Solution
State 1
Refer to Table A-12, obtain the value of saturated temperature at a pressure of 320 kPa as
The given temperature in state 1 is less than the saturated temperature at a pressure of 320 kPa.
Hence, state 1 is compressed liquid.
As wee see now there is no data for compressed liquid water in table A-7 for pressure 320 kPa, so calculate the specific internal energy and specific volume of a mixture at a saturated refrigerant-134a at a temperature of
State 2
Refer to Table A-4, obtain the specific volume at saturated liquid and specific internal energy at saturated liquid at a temperature of
Thus, the state 2 condition is saturated liquid.
State 3
Refer to Table A-13, “Superheater refrigerant-134a”, obtain the pressure and specific internal energy at a temperature and specific volume of
The given specific internal energy is greater than the specific internal energy at saturated vapour at a pressure of 140 kPa refer from Table A-12.
Thus, state 3 is a superheated steam.
State 4
Refer to Table A-12, obtain the specific volume and specific internal energy at saturated liquid
As we see now the given specific volume of the mixture
Hence, the state 4 is known as saturated mixture.
Refer to Table A-12, obtain the temperature at a pressure of 180 kPa as
Calculate the quality at state 1.
Substitute
Calculate the specific internal state.
Here, specific internal energy at saturated liquid and saturated vapour is
Substitute
State 5
Since
Thus, the state 5 is superheated steam.
Convert the unit of pressure from kPa to MPa.
Refer to Table A-13, obtain the temperature and specific volume at a pressure of 0.20 MPa and specific intenal energy of 249 kJ/kg as
From the above calculations and referred from the steam table, complete the table of
| P, kPa | u, kJ/kg | x, quality | Phase description | ||
| 320 | -12 | --- | compressed liquid | ||
| 1000 | 39.37 | -- | saturated liquid | ||
| 40 | 1.17794 | - | superheated steam | ||
| 180 | 0.0700 | saturated mixture | |||
| 200 | 249 | -- | superheated steam |
Want to see more full solutions like this?
Chapter 3 Solutions
THERMODYNAMICS LLF W/ CONNECT ACCESS
- This is an exam review question. The answer is Pmin = 622.9 lb but whyarrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardPlease do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forward
- Please do not use any AI tools to solve this question. I need a fully manual, step-by-step solution with clear explanations, as if it were done by a human tutor. No AI-generated responses, please.arrow_forwardThis is an old practice exam. Fce = 110lb and FBCD = 62 lb but whyarrow_forwardQuiz/An eccentrically loaded bracket is welded to the support as shown in Figure below. The load is static. The weld size for weld w1 is h1 = 4mm, for w2 h2 = 6mm, and for w3 is h3 =6.5 mm. Determine the safety factor (S.f) for the welds. F=29 kN. Use an AWS Electrode type (E100xx). 163 mm S 133 mm 140 mm Please solve the question above I solved the question but I'm sure the answer is wrong the link : https://drive.google.com/file/d/1w5UD2EPDiaKSx3W33aj Rv0olChuXtrQx/view?usp=sharingarrow_forward
- Q2: (15 Marks) A water-LiBr vapor absorption system incorporates a heat exchanger as shown in the figure. The temperatures of the evaporator, the absorber, the condenser, and the generator are 10°C, 25°C, 40°C, and 100°C respectively. The strong liquid leaving the pump is heated to 50°C in the heat exchanger. The refrigerant flow rate through the condenser is 0.12 kg/s. Calculate (i) the heat rejected in the absorber, and (ii) the COP of the cycle. Yo 8 XE-V lo 9 Pc 7 condenser 5 Qgen PG 100 Qabs Pe evaporator PRV 6 PA 10 3 generator heat exchanger 2 pump 185 absorberarrow_forwardQ5:(? Design the duct system of the figure below by using the balanced pressure method. The velocity in the duct attached to the AHU must not exceed 5m/s. The pressure loss for each diffuser is equal to 10Pa. 100CFM 100CFM 100CFM ☑ ☑ 40m AHU -16m- 8m- -12m- 57m 250CFM 40m -14m- 26m 36m ☑ 250CFMarrow_forwardA mass of ideal gas in a closed piston-cylinder system expands from 427 °C and 16 bar following the process law, pv1.36 = Constant (p times v to the power of 1.36 equals to a constant). For the gas, initial : final pressure ratio is 4:1 and the initial gas volume is 0.14 m³. The specific heat of the gas at constant pressure, Cp = 0.987 kJ/kg-K and the specific gas constant, R = 0.267 kJ/kg.K. Determine the change in total internal energy in the gas during the expansion. Enter your numerical answer in the answer box below in KILO JOULES (not in Joules) but do not enter the units. (There is no expected number of decimal points or significant figures).arrow_forward
- my ID# 016948724. Please solve this problem step by steparrow_forwardMy ID# 016948724 please find the forces for Fx=0: fy=0: fz=0: please help me to solve this problem step by steparrow_forwardMy ID# 016948724 please solve the proble step by step find the forces fx=o: fy=0; fz=0; and find shear moment and the bending moment diagran please draw the diagram for the shear and bending momentarrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning Automotive Technology: A Systems Approach (MindTa...Mechanical EngineeringISBN:9781133612315Author:Jack Erjavec, Rob ThompsonPublisher:Cengage Learning
Automotive Technology: A Systems Approach (MindTa...Mechanical EngineeringISBN:9781133612315Author:Jack Erjavec, Rob ThompsonPublisher:Cengage Learning Welding: Principles and Applications (MindTap Cou...Mechanical EngineeringISBN:9781305494695Author:Larry JeffusPublisher:Cengage Learning
Welding: Principles and Applications (MindTap Cou...Mechanical EngineeringISBN:9781305494695Author:Larry JeffusPublisher:Cengage Learning



