![OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305673939
Author: Darrell Ebbing; Steven D. Gammon
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.7, Problem 3.4CC
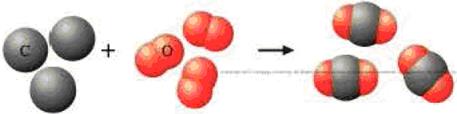
The main reaction of a charcoal grill is C(s) + O2(g) → CO2(g). Which of the statements below are incorrect? Why?
- a 1 atom of carbon reacts with 1 molecule of oxygen to produce 1 molecule of CO2.
- b 1 g of C reacts with 1 g of O2 to produce 2 grams of CO2.
- c 1 g of C reacts with 0.5 g of O2 to produce 1 g of CO2.
- d 12 g of C reacts with 32 g of O2 to produce 44 g of CO2.
- e 1 mol of C reacts with 1 mol of O2 to produce 1 mol of CO2.
- f 1 mol of C reacts with 0.5 mol of O2 to produce 1 mol of CO2.
- g

- h
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you explain these two problems for me
个
^
Blackboard
x Organic Chemistry II Lecture (m x
Aktiv Learning App
x
→ C
app.aktiv.com
←
Curved arrows are used to illustrate the flow of electrons. Using
the provided starting and product structures, draw the curved
electron-pushing arrows for the following reaction or
mechanistic step(s).
Be sure to account for all bond-breaking and bond-making
steps.
Problem 28 of 35
:OH H
HH
KO
Select to Edit Arrows
CH CH₂OK, CH CH2OH
5+
H
:0:
Done
Can you explain those two problems for me please.
Chapter 3 Solutions
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 3.1 - Calculate the formula weights of the following...Ch. 3.1 - For the following compounds, write the molecular...Ch. 3.2 - a. What is the mass in grams of a calcium atom,...Ch. 3.2 - Hydrogen peroxide, H2O2, is a colorless liquid. A...Ch. 3.2 - Nitric acid, HNO3, is a colorless, corrosive...Ch. 3.2 - Hydrogen cyanide, HCN, is a volatile, colorless...Ch. 3.2 - You have 1.5 moles of tricycles. a How many moles...Ch. 3.3 - Ammonium nitrate, NH4NO3, which is prepared from...Ch. 3.3 - How many grams of nitrogen, N, are there in a...Ch. 3.4 - A 3.87-mg sample of ascorbic acid (vitamin C)...
Ch. 3.4 - Prob. 3.2CCCh. 3.5 - A sample of compound weighing 83.5 g contains 33.4...Ch. 3.5 - Benzoic acid is a white, crystalline powder used...Ch. 3.5 - The percentage composition of acetaldehyde is...Ch. 3.5 - Prob. 3.3CCCh. 3.6 - In an industrial process, hydrogen chloride, HCl,...Ch. 3.7 - Sodium is a soft, reactive metal that instantly...Ch. 3.7 - Sphalerite is a zinc sulfide (ZnS) mineral and an...Ch. 3.7 - The British chemist Joseph Priestley prepared...Ch. 3.7 - The main reaction of a charcoal grill is C(s) +...Ch. 3.8 - Solid ReO3 is a material that is an extremely good...Ch. 3.8 - Aluminum chloride, AlCl3, is used as a catalyst in...Ch. 3.8 - In an experiment, 7.36 g of zinc was heated with...Ch. 3.8 - New industrial plants for acetic acid react liquid...Ch. 3.8 - Prob. 3.6CCCh. 3 - What is the difference between a formula weight...Ch. 3 - Describe in words how to obtain the formula weight...Ch. 3 - One mole of N2 contains how many N2 molecules? How...Ch. 3 - Prob. 3.4QPCh. 3 - Prob. 3.5QPCh. 3 - A substance has the molecular formula C6H12O2....Ch. 3 - Hydrogen peroxide has the empirical formula HO and...Ch. 3 - Describe in words the meaning of the equation...Ch. 3 - Prob. 3.9QPCh. 3 - Prob. 3.10QPCh. 3 - Prob. 3.11QPCh. 3 - Prob. 3.12QPCh. 3 - How many grams of NH3 will have the same number of...Ch. 3 - Which of the following has the largest number of...Ch. 3 - How many atoms are present in 123 g of magnesium...Ch. 3 - Calculations with Chemical Formulas and Equations...Ch. 3 - Prob. 3.17QPCh. 3 - Moles within Moles and Molar Mass Part 1: a How...Ch. 3 - You react nitrogen and hydrogen in a container to...Ch. 3 - Propane, C3H8, is the fuel of choice in a gas...Ch. 3 - Prob. 3.21QPCh. 3 - Prob. 3.22QPCh. 3 - High cost and limited availability of a reactant...Ch. 3 - Prob. 3.24QPCh. 3 - A friend asks if you would be willing to check...Ch. 3 - Prob. 3.26QPCh. 3 - Find the formula weights of the following...Ch. 3 - Find the formula weights of the following...Ch. 3 - Calculate the formula weight of the following...Ch. 3 - Calculate the formula weight of the following...Ch. 3 - Ammonium nitrate, NH4NO3, is used as a nitrogen...Ch. 3 - Phosphoric acid, H3PO4, is used to make phosphate...Ch. 3 - Calculate the mass (in grams) of each of the...Ch. 3 - Diethyl ether, (C2H5)2O, commonly known as ether,...Ch. 3 - Glycerol, C3H8O3, is used as a moistening agent...Ch. 3 - Calculate the mass in grams of the following. a...Ch. 3 - Calculate the mass in grams of the following. a...Ch. 3 - Boric acid, H3BO3, is a mild antiseptic and is...Ch. 3 - Carbon disulfide, CS2, is a colorless, highly...Ch. 3 - Obtain the moles of substance in the following. a...Ch. 3 - Obtain the moles of substance in the following. a...Ch. 3 - Calcium sulfate, CaSO4, is a white, crystalline...Ch. 3 - A 1.547-g sample of blue copper(II) sulfate...Ch. 3 - Calculate the following. a number of atoms in 8.21...Ch. 3 - Calculate the following. a number of atoms in 25.7...Ch. 3 - Carbon tetrachloride is a colorless liquid used in...Ch. 3 - Chlorine trifluoride is a colorless, reactive gas...Ch. 3 - A 1.680-g sample of coal contains 1.584 g C....Ch. 3 - A 6.01-g aqueous solution of isopropyl alcohol...Ch. 3 - Phosphorus oxychloride is the starting compound...Ch. 3 - Ethyl mercaptan is an odorous substance added to...Ch. 3 - A fertilizer is advertised as containing 14.0%...Ch. 3 - Seawater contains 0.0065% (by mass) of bromine....Ch. 3 - A sample of an alloy of aluminum contains 0.0898...Ch. 3 - A sample of gas mixture from a neon sign contains...Ch. 3 - Calculate the percentage composition for each of...Ch. 3 - Calculate the percentage composition for each of...Ch. 3 - Calculate the mass percentage of each element in...Ch. 3 - Calculate the mass percentage of each element in...Ch. 3 - Which contains more carbon, 6.01 g of glucose....Ch. 3 - Which contains more sulfur, 40.8 g of calcium...Ch. 3 - Ethylene glycol is used as an automobile...Ch. 3 - Prob. 3.64QPCh. 3 - An oxide of osmium (symbol Os) is a pale yellow...Ch. 3 - An oxide of tungsten (symbol W) is a bright yellow...Ch. 3 - Potassium bromate is a colorless, crystalline...Ch. 3 - Hydroquinone, used as a photographic developer, is...Ch. 3 - Acrylic acid, used in the manufacture of acrylic...Ch. 3 - Malonic acid is used in the manufacture of...Ch. 3 - Two compounds have the same composition: 92.25% C...Ch. 3 - Two compounds have the same composition: 85.62% C...Ch. 3 - Putreseine a substance produced by decaying...Ch. 3 - Compounds of boron with hydrogen are called...Ch. 3 - Oxalic acid is a toxic substance used by laundries...Ch. 3 - Adipic acid is used in the manufacture of nylon....Ch. 3 - Ethylene, C2H4, bums in oxygen to give carbon...Ch. 3 - Hydrogen sulfide gas, H2S, burns in oxygen to give...Ch. 3 - Prob. 3.79QPCh. 3 - Ethanol, C2H5OH, burns with the oxygen in air to...Ch. 3 - Iron in the form of fine wire burns in oxygen to...Ch. 3 - Prob. 3.82QPCh. 3 - Nitric acid, HNO3, is manufactured by the Ostwald...Ch. 3 - White phosphorus, P4, is prepared by fusing...Ch. 3 - Tungsten metal, W, is used to make incandescent...Ch. 3 - Acrylonitrile, C3H3N, is the starting material for...Ch. 3 - The following reaction, depicted using molecular...Ch. 3 - Using the following reaction (depicted using...Ch. 3 - When dinitrogen pentoxide, N2O5, a white solid, is...Ch. 3 - Copper metal reacts with mine acid. Assume that...Ch. 3 - Potassium superoxide, KO2, is used in rebreathing...Ch. 3 - Solutions of sodium hypochlorite, NaClO, are sold...Ch. 3 - Methanol, CH3OH, is prepared industrially from the...Ch. 3 - Carbon disulfide, CS2, burns in oxygen. Complete...Ch. 3 - Prob. 3.95QPCh. 3 - Hydrogen cyanide, HCN, is prepared from ammonia,...Ch. 3 - Aspirin (acetylsalicylic acid) is prepared by...Ch. 3 - Methyl salicylate (oil of wintergreen) is prepared...Ch. 3 - Caffeine, the stimulant in coffee and tea, has the...Ch. 3 - Morphine, a narcotic substance obtained from...Ch. 3 - A moth repellent, para-dichlorobenzene, has the...Ch. 3 - Sorbic acid is added to food as a mold inhibitor....Ch. 3 - Thiophene is a liquid compound of the elements C,...Ch. 3 - Aniline, a starting compound for urethane plastic...Ch. 3 - A sample of limestone (containing calcium...Ch. 3 - A titanium ore contains rutile (TiO2) plus some...Ch. 3 - Ethylene oxide, C2H4O, is made by the oxidation of...Ch. 3 - Nitrobenzene, C6H5NO2, an important raw material...Ch. 3 - Zinc metal can be obtained from zinc oxide, ZnO,...Ch. 3 - Hydrogen cyanide, HCN, can be made by a two-step...Ch. 3 - Calcium carbide, CaC2, used to produce acetylene,...Ch. 3 - A mixture consisting of 11.9 g of calcium...Ch. 3 - Alloys, or metallic mixtures, of mercury with...Ch. 3 - A sample of sandstone consists of silica, SiO2,...Ch. 3 - Prob. 3.115QPCh. 3 - Prob. 3.116QPCh. 3 - Exactly 4.0 g of hydrogen gas combines with 32 g...Ch. 3 - Aluminum metal reacts with iron(III) oxide to...Ch. 3 - Prob. 3.119QPCh. 3 - You perform a combustion analysis on a 255 mg...Ch. 3 - Prob. 3.121QPCh. 3 - A 3.0-L sample of paint that has a density of...Ch. 3 - A 12.1-g sample of Na2SO3 is mixed with a 14.6-g...Ch. 3 - Potassium superoxide, KO2, is employed in a...Ch. 3 - Calcium carbonate is a common ingredient in...Ch. 3 - Prob. 3.126QPCh. 3 - Prob. 3.127QPCh. 3 - Copper reacts with nitric acid according to the...Ch. 3 - A sample of methane gas, CH4(g), is reacted with...Ch. 3 - A sample containing only boron and fluorine was...Ch. 3 - Prob. 3.131QPCh. 3 - Prob. 3.132QPCh. 3 - A 0.500-g mixture of Cu2O and CuO contains 0.425 g...Ch. 3 - A mixture of Fe2O3, and FeO was found to contain...Ch. 3 - Hemoglobin is the oxygen-carrying molecule of red...Ch. 3 - Penicillin V was treated chemically to convert...Ch. 3 - A 3.41-g sample of a metallic element, M, reacts...Ch. 3 - Prob. 3.138QPCh. 3 - An alloy of iron (54.7%), nickel (45.0%), and...Ch. 3 - Prob. 3.140QPCh. 3 - A power plant is driven by the combustion of a...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Define equilibrium population. Outline the conditions that must be met for a population to stay in genetic e...
Biology: Life on Earth (11th Edition)
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
2. Why is it that the range of resting blood pressures of humans is best represented by a bell-shaped curve co...
Human Biology: Concepts and Current Issues (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Do we need to draw the "ethyne" first for this problem? im confusedarrow_forwardCan you explain how this problem was solved.arrow_forwardQuestion 2 show work. don't Compound give Ai generated solution So (J K-1 mol-1) A 26 B 54 C 39 D 49 At 298 K, AG° is 375 kJ for the reaction 1A + 1B → 4C + 2D Calculate AH° for this reaction in kJ.arrow_forward
- 1. Provide a complete IUPAC name for each of the following compounds. a) b) c) OH OH OH a) b) c) 2. Provide a complete IUPAC name for each of the following compounds. a) b) a) OH b) он c) OB >=arrow_forwardc) 3. Provide a common name for each of the following alcohols. a) a) OH b) OH c) HO b) c) 4. Provide a common name for each of the following compounds. b) OH a) 5 a) Y OH c) OHarrow_forwardUsing the critical constants for water (refer to the table in the lecture slides), calculate the second virial coefficient. Assume that the compression factor (Z) is expressed as an expansion series in terms of pressure.arrow_forward
- +3413 pts /4800 Question 38 of 48 > Write the full electron configuration for a Kion. © Macmillan Learning electron configuration: ↓ Resources Solution Penalized → Al Tutor Write the full electron configuration for an Fion. electron configuration: T G 6 & 7 Y H כ Y 00 8 hp 9 J K no L 144 P 112 | t KC 47°F Clear ins prt sc delete ] backspace erarrow_forwardHow to solve these types of problems step by step? I'm so confused.arrow_forwardIdentify the expected product of the following Claisen rearrangement. || = IV OV 00000 5 ОН Он Он Он Он || III IV Varrow_forward
- Can you please color-code and explain how to solve this and any molecular orbital diagram given? I'm so confused; could you provide baby steps regardless of which problem type they gave me?arrow_forwardConsider the following structure. OH Esmolol The synthesis of this compound uses a building block derived from either ethylene oxide or epichlorohydrin. 1) Determine which building block was used: | 2) Draw the structure of the nucleophiles that were used along with this building block in the synthesis of the molecule. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. You do not have to consider stereochemistry. Θε {n [arrow_forward< 10:44 5GW 10 Question 7/8 Show Answer Convert 46.0 mm to inches (1 inch = 2.54 cm) 46.0 DAM STARTING AMOUNT 1 cm 1 in 46.0 mm x ☑ 10 mm 10 cm ADD FACTOR DELETE x() X × = 1.81 in = 1 10 Dam ANSWER RESET ១ 2.54 0.0460 mm 10 1000 in 0.001 11.7 m 4.60 18.1 cm 100 1.81 0.394 1 0.1 46.0 0.01 Tap here for additional resourcesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY