
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
4th Edition
ISBN: 8220102737037
Author: Klein
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.4, Problem 3.27P
Predict the multiplicity of each signal in the expected proton NMR spectrum of each of the following compounds:
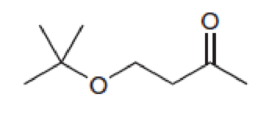
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Organic bases have lone pairs of electrons that are capable of accepting protons. Lone pair electrons in a neutral or negatively charged species, or pi electron pairs. Explain the latter case (pi electron pairs).
Describe the propyl anion.
Indicate the names of these compounds (if they exist).
0:
HỌC—NH
CH3CH2-CH2
Chapter 3 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 3.1 - Prob. 3.2PCh. 3.1 - Prob. 3.3PCh. 3.1 - Prob. 3.4PCh. 3.1 - Prob. 3.5PCh. 3.1 - Prob. 3.6PCh. 3.1 - Prob. 3.7PCh. 3.1 - Prob. 3.8PCh. 3.1 - Prob. 3.9PCh. 3.1 - Prob. 3.10PCh. 3.1 - If you look at your answers to the previous...
Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Prob. 3.19PCh. 3.3 - Prob. 3.21PCh. 3.3 - Prob. 3.22PCh. 3.3 - Prob. 3.23PCh. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.9 - Prob. 3.43PCh. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Prob. 3.48PCh. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The data were obtained from a use-dilution test comparing four disinfectants against Salmonella choleraesuis. G...
Microbiology: An Introduction
The number of named species is about ________, but the actual number of species on Earth is estimated to be abo...
Biology: Life on Earth with Physiology (11th Edition)
Compare each of the mechanisms listed here with the mechanism for each of the two parts of the acid-catalyzed h...
Organic Chemistry (8th Edition)
Use the key to classify each of the following described tissue types into one of the four major tissue categori...
Anatomy & Physiology (6th Edition)
Dr. Ara B. Dopsis and Dr. C. Ellie Gans are performing genetic crosses on daisy plants. They self-fertilize a b...
Genetic Analysis: An Integrated Approach (3rd Edition)
Steam at 500kPa,300C is used to heat cold water at 15°C to 75°C for a domestic hot water supply. How much steam...
Fundamentals Of Thermodynamics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- N Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forwardName these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forward
- HH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forwardName these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forward
- Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forwardWhich molecule is the most stable? Please explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY