
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
4th Edition
ISBN: 8220102737037
Author: Klein
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.10, Problem 3.54P
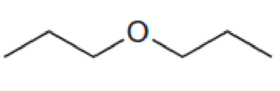
For each compound below, predict the number of signals and the location of each signal in the expected 13C NMR spectrum.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Question 10
1 pts
Which of the following is the most accurate nomenclature?
1-hydroxy-1-methyldecane-4,7-dione
2-hydroxy-2-methyldecane-5,8-dione
4,6-dioxo-2-methyldecane-2-ol
9-hydroxy-9-methyldecane-3,6-dione
8-hydroxy-8-methylnonane-3,6-dione
OH
Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.
What are the most proper reagents to achieve these products?
سد
1.
2.
OH
○ 1. BrMgC6H6; 2. H+
○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+
O 1. CH3CH2CHO; 2. H+
O 1. BrMgCH2CH3; 2. H+
Chapter 3 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
Ch. 3.1 - Prob. 3.2PCh. 3.1 - Prob. 3.3PCh. 3.1 - Prob. 3.4PCh. 3.1 - Prob. 3.5PCh. 3.1 - Prob. 3.6PCh. 3.1 - Prob. 3.7PCh. 3.1 - Prob. 3.8PCh. 3.1 - Prob. 3.9PCh. 3.1 - Prob. 3.10PCh. 3.1 - If you look at your answers to the previous...
Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Predict the chemical shifts for the signals in the...Ch. 3.2 - Prob. 3.19PCh. 3.3 - Prob. 3.21PCh. 3.3 - Prob. 3.22PCh. 3.3 - Prob. 3.23PCh. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.4 - Predict the multiplicity of each signal in the...Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.5 - Below are NMR spectra of several compounds....Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.8 - Calculate the degree of unsaturation for each of...Ch. 3.9 - Prob. 3.43PCh. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Propose a structure for a compound with molecular...Ch. 3.9 - Prob. 3.48PCh. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...Ch. 3.10 - For each compound below, predict the number of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Where is each type of membrane located in the body? What are their functions?
Principles of Anatomy and Physiology
Lewis structure for lonic compounds
35. Is each compound best represented by an ionic or a covalent Lewis stru...
Introductory Chemistry (6th Edition)
19. What are the strength and direction of the electric field 2.0 cm from a small glass bead that has been char...
College Physics: A Strategic Approach (3rd Edition)
37. Consider the reaction:
Complete the table. Assume that all concentrations are equilibrium concentrat...
Chemistry: Structure and Properties (2nd Edition)
Name each of the following:
Organic Chemistry (8th Edition)
Why is turbidity not an accurate measurement of viable bacteria in a culture?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forwardPlease use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forward
- Indicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forwardPredict the major products of the following organic reaction: + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardPolar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solventsarrow_forward
- Deducing the Peactants Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Xarrow_forwardDraw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)arrow_forwardBookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY