
Applied Physics
11th Edition
ISBN: 9780132719865
Author: EWEN, Dale
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.3, Problem 20P
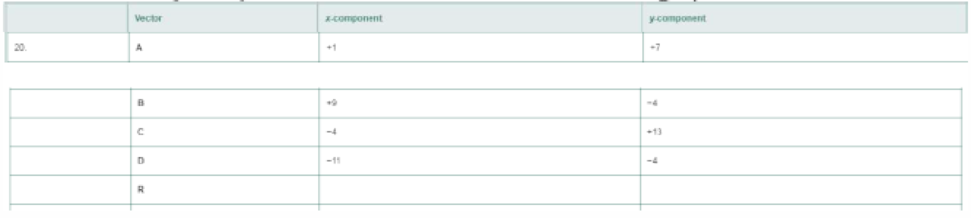
Find the x- and y- components of each resultant vector R and graph the resultant vector R
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path
function, and in this problem, you will show an example of the entropy change for an ideal gas
being the same when you go between the same two states by two different pathways.
A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to
V2, at fixed particle number N and energy, U.
B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number
from N₁ to N2, at fixed volume V and energy U.
C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) →
(V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle
number changes at fixed volume. Again, assume energy is constant.
Please don't use Chatgpt will upvote and give handwritten solution
6. We used the constant volume heat capacity, Cv, when we talked about
thermodynamic cycles. It acts as a proportionality constant between energy and
temperature: dU = C₁dT.
You can also define a heat capacity for constant pressure processes, Cp. You can think
of enthalpy playing a similar role to energy, but for constant pressure processes
δαρ
C = (37) - Sup
Ср
ат
P
=
ат
Starting from the definition of enthalpy, H = U + PV, find the relationship between Cy
and Cp for an ideal gas.
Chapter 3 Solutions
Applied Physics
Ch. 3.1 - Using the scale 1.0 cm = 50km, find the length of...Ch. 3.1 - Using the scale 1.0 cm = 50km, find the length of...Ch. 3.1 - Using the scale 1.0 cm = 50km, find the length of...Ch. 3.1 - Using the scale 1.0 cm = 50km, find the length of...Ch. 3.1 - Using the scale 1.0 cm = 50km, find the length of...Ch. 3.1 - Using the scale 1.0 cm = 50km, find the length of...Ch. 3.1 - Draw the vectors in Problems 1 through 6 using the...Ch. 3.1 - Draw the vectors in Problems 1 through 6 using the...Ch. 3.1 - Draw the vectors in Problems 1 through 6 using the...Ch. 3.1 - Draw the vectors in Problems 1 through 6 using the...
Ch. 3.1 - Draw the vectors in Problems 1 through 6 using the...Ch. 3.1 - Draw the vectors in Problems 1 through 6 using the...Ch. 3.1 - Using the scale 14 in. = 20 mi, find the length of...Ch. 3.1 - Using the scale 14 in. = 20 mi, find the length of...Ch. 3.1 - Using the scale 14 in. = 20 mi, find the length of...Ch. 3.1 - Using the scale 14 in. = 20 mi, find the length of...Ch. 3.1 - Using the scale 14 in. = 20 mi, find the length of...Ch. 3.1 - Using the scale 14 in. = 20 mi, find the length of...Ch. 3.1 - Draw the vectors in Problems 13 through 18 using...Ch. 3.1 - Draw the vectors in Problems 13 through 18 using...Ch. 3.1 - Draw the vectors in Problems 13 through 18 using...Ch. 3.1 - Draw the vectors in Problems 13 through 18 using...Ch. 3.1 - Draw the vectors in Problems 13 through 18 using...Ch. 3.1 - Draw the vectors in Problems 13 through 18 using...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Find the x- and y-components of each vector in the...Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Make a sketch of each vector in standard position....Ch. 3.2 - Find the x- and y- components of each vector. 19.Ch. 3.2 - Find the x- and y- components of each vector. 20.Ch. 3.2 - Find the x- and y- components of each vector. 21.Ch. 3.2 - Find the x- and y- components of each vector. 22.Ch. 3.2 - Find the x- and y- components of each vector. 23.Ch. 3.2 - Find the x- and y- components of each vector. 24.Ch. 3.2 - Find the x- and y-components of each vector given...Ch. 3.2 - Find the x- and y-components of each vector given...Ch. 3.2 - Find the x- and y-components of each vector given...Ch. 3.2 - Find the x- and y-components of each vector given...Ch. 3.2 - Find the x- and y-components of each vector given...Ch. 3.2 - Find the x- and y-components of each vector given...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant or each...Ch. 3.3 - Use graph paper to find the resultant or each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Use graph paper to find the resultant of each...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - Find the x- and y- components of each resultant...Ch. 3.3 - For each set of vectors, graph and find the x- and...Ch. 3.3 - For each set of vectors, graph and find the x- and...Ch. 3.3 - For each set of vectors, graph and find the x- and...Ch. 3.3 - For each set of vectors, graph and find the x- and...Ch. 3.3 - For each set of vectors, graph and find the x- and...Ch. 3.3 - For each set of vectors, graph and find the x- and...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, rind each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - In Problems 31 through 42, find each resultant...Ch. 3.3 - A road grader must go around a pond by traveling...Ch. 3.3 - An earthmover must go north 350 m and then west...Ch. 3.3 - An airplane takes off and flies 225 km on a course...Ch. 3.3 - A ship travels 50.0 mi on a course of 15.0 south...Ch. 3.3 - A ship travels 135 km from port on a course of...Ch. 3.3 - A ship travels 145 km from port on a course of...Ch. 3 - Displacement a. can be interchanged with...Ch. 3 - When adding vectors, the order in which they are...Ch. 3 - A vector is in standard position when its initial...Ch. 3 - Discuss number plane, origin, and axis in your own...Ch. 3 - Can every vector be described in terms of its...Ch. 3 - Describe how to add two or more vectors...Ch. 3 - Describe how to find a resultant vector if given...Ch. 3 - Is a vector limited to a single position in the...Ch. 3 - Is the angle of a vector in standard position...Ch. 3 - What are the limits on the angle measure of a...Ch. 3 - Describe how to find the x- and y-components of a...Ch. 3 - Describe how to find a vector in standard position...Ch. 3 - Find the x- and y-components of vector R, which...Ch. 3 - Find the x- and y-components of vector R, which...Ch. 3 - Find the x- and y-components of vector R, which...Ch. 3 - Vector R has length 9.00 cm at 240.0. Find its x-...Ch. 3 - Vector R has length 9.00 cm at 40.0. Find its x-...Ch. 3 - Vector R has length 18.0 cm at 305.0. Find its x-...Ch. 3 - A hiker is plotting his course on a map with a...Ch. 3 - A hiker is plotting his course on a map with a...Ch. 3 - A co-pilot is charting her course on a map with a...Ch. 3 - A co-pilot is charting her course on a map with a...Ch. 3 - Vector R has x-component = +14.0 and y-component =...Ch. 3 - Vector R has x-component = -5.00 and y-component =...Ch. 3 - Vector R has x-component = +8.00 and y-component =...Ch. 3 - Vector R has x-component = -3.00 and y-component =...Ch. 3 - Vectors A, B, and C are given. Vector A has...Ch. 3 - Vectors A, B, and C are given. Vector A has...Ch. 3 - Vectors A, B. and C are given. Vector A has...Ch. 3 - Vectors A, B, and C are given. Vector A has...Ch. 3 - Graph and find x- and y-components of each...Ch. 3 - Graph and find the x- and y-components of each...Ch. 3 - An airplane takes off and flies 245 km on a course...Ch. 3 - A ship travels 155 km from port on a course of...Ch. 3 - The New Clark Bridge is an elegant cable-stayed...Ch. 3 - Frank just learned that the 800-m section of...Ch. 3 - Power cables need to be suspended by the power...Ch. 3 - With the airplane cruising at 30,000 ft, the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
What percentage of Earths land surface do glaciers presently cover? ____________
Applications and Investigations in Earth Science (9th Edition)
What is the difference between cellular respiration and external respiration?
Human Physiology: An Integrated Approach (8th Edition)
Draw the enol tautomers for each of the following compounds. For compounds that have more than one enol tautome...
Organic Chemistry (8th Edition)
10. In rats, gene produces black coat color if the genotype is, but black pigment is not produced if the genoty...
Genetic Analysis: An Integrated Approach (3rd Edition)
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
Complete and balance each acid-base reaction. a. HC2H3O2(aq)+Ca(OH)2(aq) b. HBr(aq)+LiOH(aq) c. H2SO4(aq)+Ba(OH...
Introductory Chemistry (6th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Pure membranes of dipalmitoyl lecithin phospholipids are models of biological membranes. They melt = 41°C. Reversible melting experiments indicate that at Tm AHm=37.7 kJ mol-1. Calculate: A. The entropy of melting, ASm- B. The Gibbs free energy of melting, AGm- C. Does the membrane become more or less ordered upon melting? D. There are 32 rotatable CH2 CH2 bonds in each molecule that can rotate more freely if the membrane melts. What is the increase in multiplicity on melting a mole of bonds?arrow_forward5. Heat capacity often has a temperature dependence for real molecules, particularly if you go over a large temperature range. The heat capacity for liquid n-butane can be fit to the equation Cp(T) = a + bT where a = 100 J K₁₁ mol¹ and b = 0.1067 J K² mol¹ from its freezing point (T = 140 K) to its boiling point (T₁ = 270 K). A. Compute AH for heating butane from 170 K to 270 K. B. Compute AS for the same temperature range.arrow_forward4. How much energy must be transferred as heat to cause the quasi-static isothermal expansion of one mole of an ideal gas at 300 K from PA = 1 bar to PB = 0.5 bar? A. What is VA? B. What is VB? C. What is AU for the process? D. What is AH for the process? E. What is AS for the process?arrow_forward
- 1. The diagram shows the tube used in the Thomson experiment. a. State the KE of the electrons. b. Draw the path of the electron beam in the gravitational field of the earth. C. If the electric field directed upwards, deduce the direction of the magnetic field so it would be possible to balance the forces. electron gun 1KVarrow_forwardas a hiker in glacier national park, you need to keep the bears from getting at your food supply. You find a campground that is near an outcropping of ice. Part of the outcropping forms a feta=51.5* slopeup that leads to a verticle cliff. You decide that this is an idea place to hang your food supply out of bear reach. You put all of your food into a burlap sack, tie a rope to the sack, and then tie a bag full of rocks to the other end of the rope to act as an anchor. You currently have 18.5 kg of food left for the rest of your trip, so you put 18.5 kg of rocks in the anchor bag to balance it out. what happens when you lower the food bag over the edge and let go of the anchor bag? Determine the acceleration magnitude a of the two-bag system when you let go of the anchor bag?arrow_forward2. A thin Nichrome wire is used in an experiment to test Ohm's law using a power supply ranging from 0 to 12 V in steps of 2 V. Why isn't the graph of I vs V linear? 1. Nichrome wire does obey Ohm's law. Explain how that can that be true given the results abovearrow_forward
- 1. The average KE and temperature in Kelvin of the molecules of a gas are related by the equation KE = 3/2 KT where k is the Boltzmann constant 1.38 x 10 m² kg s². The diagram shows the energy levels for a Hydrogen atom. Energy/eV 0.00 -1.51 3.39 13.58 Use this information to show that Hydrogen at room temperature will not emit light. 2. When hydrogen burns in oxygen 241.8 kJ of energy are released per mole. Show that this reaction can produce light.arrow_forward3. By using the fact that around any closed loop the sum of the EMFS = the sum of the PDs. Write equations for the two loops shown in the cct below. 40 ΔΩ I₂ 4V (loop1 20 (loop2) 2v I+12 Use these equations to show that the current flowing through the 20 resistor is 0.75Aarrow_forward5. A potential divider circuit is made by stretching a 1 m long wire with a resistance of 0.1 per cm from A to B as shown. 8V A 100cm B sliding contact 5Ω A varying PD is achieved across the 5 Q resistor by moving the slider along the resistance wire. Calculate the distance from A when the PD across the 5 Q resistor is 6 V.arrow_forward
- 4. A voltmeter with resistance 10 kQ is used to measure the pd across the 1 kQ resistor in the circuit below. 6V 5ΚΩ 1ΚΩ V Calculate the percentage difference between the value with and without the voltmeter.arrow_forward1. A 9V battery with internal resistance 5 2 is connected to a 100 2 resistor. Calculate: a. the Power dissipated in the 100 2 resistor b. The heat generated per second inside the battery. C. The rate of converting chemical to electrical energy by the battery. 2. A 230 V kettle is rated at 1800 W. Calculate the resistance of the heating element.arrow_forward2. If each of the resistors in the circuit below has resistance R show that the total resistance between A and B is 5R/11 A Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Introduction to Vectors and Their Operations; Author: Professor Dave Explains;https://www.youtube.com/watch?v=KBSCMTYaH1s;License: Standard YouTube License, CC-BY