
EBK ORGANIC CHEMISTRY
9th Edition
ISBN: 8220100591310
Author: McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31.SE, Problem 16MP
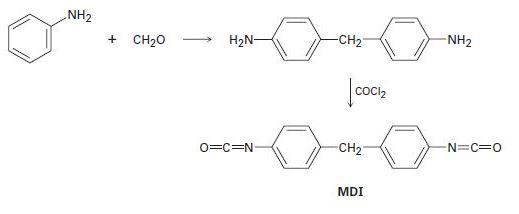
The polyurethane foam used for home insulation uses methane-diphenyldiisocyanate (MDI) as monomer. The MDI is prepared by acid-catalyzed reaction of aniline with formaldehyde, followed by treatment with phosgene, COCl2. Propose mechanisms for both steps.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
#1. Retro-Electrochemical Reaction: A ring has been made, but the light is causing the molecule to un-
cyclize. Undo the ring into all possible molecules. (2pts, no partial credit)
hv
Don't used Ai solution
I have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."
Chapter 31 Solutions
EBK ORGANIC CHEMISTRY
Ch. 31.1 - Order the following monomers with respect to their...Ch. 31.1 - Order the following monomers with respect to their...Ch. 31.1 - Prob. 3PCh. 31.2 - Prob. 4PCh. 31.2 - Prob. 5PCh. 31.3 - Prob. 6PCh. 31.3 - Prob. 7PCh. 31.4 - Prob. 8PCh. 31.4 - Show the mechanism of the nucleophilic addition...Ch. 31.5 - Prob. 10P
Ch. 31.6 - Prob. 11PCh. 31.6 - Prob. 12PCh. 31.SE - Prob. 13VCCh. 31.SE - Prob. 14VCCh. 31.SE - Prob. 15MPCh. 31.SE - The polyurethane foam used for home insulation...Ch. 31.SE - Prob. 17MPCh. 31.SE - Prob. 18MPCh. 31.SE - Prob. 19MPCh. 31.SE - Identify the monomer units from which each of the...Ch. 31.SE - Prob. 21APCh. 31.SE - Draw the structure of Kodel, a polyester prepared...Ch. 31.SE - Show the structure of the polymer that results...Ch. 31.SE - Prob. 24APCh. 31.SE - Prob. 25APCh. 31.SE - 1, 3-Cyclopentadiene undergoes thermal...Ch. 31.SE - Prob. 27APCh. 31.SE - Prob. 28APCh. 31.SE - Prob. 29APCh. 31.SE - Prob. 30APCh. 31.SE - Prob. 31APCh. 31.SE - Prob. 32APCh. 31.SE - Prob. 33APCh. 31.SE - Prob. 34APCh. 31.SE - Prob. 35AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY