
COLLEGE PHYSICS
2nd Edition
ISBN: 9781711470832
Author: OpenStax
Publisher: XANEDU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31, Problem 71PE
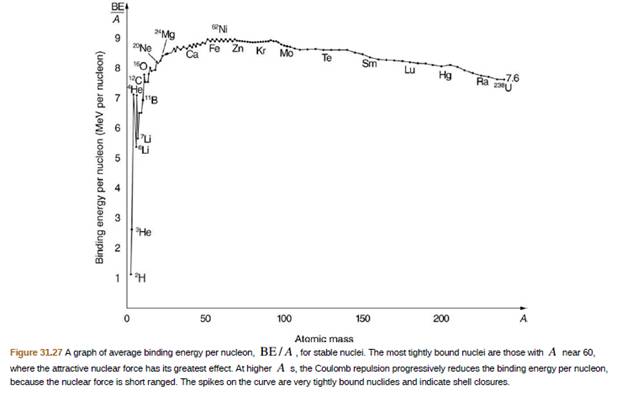
209Bi is the heaviest stable nuclide, and its BE/A is low compared with medium−mass nuclides. Calculate BE/A, the binding energy per nucleon, for 209Bi and compare it with the approximate value obtained from the graph in Figure
31.27.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Another worker is performing a task with an RWL of only 9 kg and is lifting 18 kg, giving him an LI of 2.0 (high risk).
Questions:What is the primary issue according to NIOSH?Name two factors of the RWL that could be improved to reduce risk.If the horizontal distance is reduced from 50 cm to 30 cm, how does the HM change and what effect would it have?
Two complex values are z1=8 + 8i, z2=15 + 7 i. z1∗ and z2∗ are the complex conjugate values.
Any complex value can be expessed in the form of a+bi=reiθ. Find r and θ for z1z2∗. Find r and θ for z1/z2∗? Find r and θ for (z1−z2)∗/z1+z2∗. Find r and θ for (z1−z2)∗/z1z2∗ Please explain all steps, Thank you
An ac series circuit consists of a voltage source of frequency 60 Hz and voltage amplitude V, a 505-Ω resistor, and a capacitor of capacitance 7.2 μF. What must be the source voltage amplitude V for the average electrical power consumed in the resistor to be 236 W? There is no inductance in the circuit.
Chapter 31 Solutions
COLLEGE PHYSICS
Ch. 31 - Suppose the range for 5.0 MeVa ray is known to be...Ch. 31 - What is the difference between (rays and...Ch. 31 - Ionizing radiation interacts with matter by...Ch. 31 - What characteristics of radioactivity show it to...Ch. 31 - What is the source of the energy emitted in...Ch. 31 - Consider Figure 31.3. If an electric field is...Ch. 31 - Explain how an (particle can have a larger range...Ch. 31 - Arrange the following according to their ability...Ch. 31 - Often, when people have to work around radioactive...Ch. 31 - Is it possible for light emitted by a scintillator...
Ch. 31 - The weak and strong nuclear forces are basic to...Ch. 31 - Define and make clear distinctions between the...Ch. 31 - What are isotopes? Why do different isotopes of...Ch. 31 - Star Trek fans have often heard the term...Ch. 31 - What conservation law requires an electron’s...Ch. 31 - Neutrinos are experimentally determined to have an...Ch. 31 - What do the three types of beta decay have in...Ch. 31 - In a 3109 yearold rock that originally contained...Ch. 31 - Does the number of radioactive nuclei in a sample...Ch. 31 - Radioactivity depends on the nucleus and not the...Ch. 31 - Explain how a bound system can have less mass than...Ch. 31 - Spontaneous radioactive decay occurs only when the...Ch. 31 - To obtain the most precise value of BE from the...Ch. 31 - How does the finite range of the nuclear force...Ch. 31 - Why is the number of neutrons greater than the...Ch. 31 - A physics student caught breaking conservation...Ch. 31 - When a nucleus (decays, does the (particle move...Ch. 31 - The energy of 30.0 eV is required to ionize a...Ch. 31 - A particle of ionizing radiation creates 4000 ion...Ch. 31 - (a) Repeat Exercise 31.2, and convert the energy...Ch. 31 - Suppose a particle of ionizing radiation deposits...Ch. 31 - Verify that a 2.31017kg mass of water at normal...Ch. 31 - Find the length of a side of a cube having a mass...Ch. 31 - What is the radius of an (particle?Ch. 31 - Find the radius of a 238Pu nucleus. 238Pu is a...Ch. 31 - (a) Calculate the radius of 58Ni, one of the most...Ch. 31 - The unified atomic mass unit is defined to be...Ch. 31 - What is the ratio of the velocity of a (particle...Ch. 31 - If a 1.50cmthick piece of lead can absorb 90.0% of...Ch. 31 - The detail observable using a probe is limited by...Ch. 31 - (a) Show that if you assume the average nucleus is...Ch. 31 - What is the radio of the velocity of a 5.00MeV...Ch. 31 - (a) What is the kinetic energy in MeV of a ray...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - In the following eight problems, write the...Ch. 31 - decay producing 137Ba. The parent nuclide is a...Ch. 31 - ( decay producing 90Y. The parent nuclide is a...Ch. 31 - decay producing 228Ra. The parent nuclide is...Ch. 31 - decay producing 208Pb. The parent nuclide is in...Ch. 31 - When an electron and position annihilate, both...Ch. 31 - Confirm That charge, electron family number, and...Ch. 31 - Confirm that charge, electron family number, and...Ch. 31 - Confirm that charge, electron family number, and...Ch. 31 - Confirm that charge, electron family number, and...Ch. 31 - A rare decay mode has been observed in which 222Ra...Ch. 31 - (a) Write the complete a decay equation for 226Ra....Ch. 31 - (a) Write the complete a decay equation for 249Cf....Ch. 31 - (a) Write the complete decay equation for the...Ch. 31 - (a) Write the complete decay equation for 90Sr,...Ch. 31 - Calculate the energy released in the + decay of...Ch. 31 - (a) Write the complete + decay equation for llC....Ch. 31 - (a) Calculate the energy released in the a decay...Ch. 31 - (a) Write the complete reaction equation for...Ch. 31 - (a) Write the complete reaction equation for...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - Data from the appendices and the periodic table...Ch. 31 - 2H is a loosely hound isotope of hydrogen. Called...Ch. 31 - 56Feis among the most tightly bound of all...Ch. 31 - 209Bi is the heaviest stable nuclide, and its BE/A...Ch. 31 - (a) Calculate BE/A for 235U, the rarer of the two...Ch. 31 - (a) Calculate BE/A for 12C. Stable and relatively...Ch. 31 - The fact that BE/A is greatest for A near 60...Ch. 31 - The purpose of this problem is to show in three...Ch. 31 - Unreasonable Results A particle physicist...Ch. 31 - Derive an approximate relationship between the...Ch. 31 - Integrated Concepts A 2.00T magnetic ?eld is...Ch. 31 - (a) Write the decay equation for the decay of...Ch. 31 - Unreasonable Results The relatively scarce...Ch. 31 - Unreasonable Results A physicist scatters (rays...Ch. 31 - Unreasonable Results A frazzled theoretical...Ch. 31 - Construct Your Own Problem Consider the decay of...Ch. 31 - Prob. 1TPCh. 31 - Prob. 2TPCh. 31 - Prob. 3TPCh. 31 - Prob. 4TPCh. 31 - Prob. 5TPCh. 31 - Prob. 6TPCh. 31 - Prob. 7TPCh. 31 - Prob. 8TPCh. 31 - Prob. 9TPCh. 31 - Prob. 10TPCh. 31 - Prob. 11TPCh. 31 - Prob. 12TP
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Why is the quantum-mechanical model of the atom important for understanding chemistry?
Chemistry: Structure and Properties (2nd Edition)
7. Both Tim and Jan (problem 6) have a widow’s peak (see Module 9.8), but Mike has a straight hairline. What ar...
Campbell Biology: Concepts & Connections (9th Edition)
[14.110] The following mechanism has been proposed for the gas-phase reaction of chloroform (CHCI3) and chlorin...
Chemistry: The Central Science (14th Edition)
7. Which bones form via intramembranous ossification?
a. Irregular bones
b. Certain flat bones
c. Long bones
d....
Human Anatomy & Physiology (2nd Edition)
Using the forked-line, or branch diagram, method, determine the genotypic and phenotypic ratios of these trihyb...
Concepts of Genetics (12th Edition)
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- An L−R−C series circuit has R= 280 Ω . At the frequency of the source, the inductor has reactance XLL= 905 Ω and the capacitor has reactance XC= 485 Ω . The amplitude of the voltage across the inductor is 445 V . What is the amplitude of the voltage across the resistor and the capacitor? What is the voltage amplitude of the source? What is the rate at which the source is delivering electrical energy to the circuit?arrow_forwardA 0.185 H inductor is connected in series with a 98.5 Ω resistor and an ac source. The voltage across the inductor is vL=−(12.5V)sin[(476rad/s)t]vL. Derive an expression for the voltage vR across the resistor. Express your answer in terms of the variables L, R, VL (amplitude of the voltage across the inductor), ω, and t. What is vR at 2.13 ms ? Please explain all stepsarrow_forwardA worker lifts a box under the following conditions:Horizontal distance (H): 30 cmInitial height (V): 60 cmVertical travel (D): 50 cmTorso rotation (A): 30°Frequency: 3 times/minute for 1 hourGrip: Good Question:What is the RWL for this task?What does this value mean in terms of occupational safety?arrow_forward
- Can someone helparrow_forwardCan someone help mearrow_forward3. Four identical small masses are connected in a flat perfect square. Rank the relative rotational inertias (IA, IB, IC) about the three axes of rotation shown. Axes A and B are in the plane of the square, and axis C is perpendicular to the plane, through mass m1. ΙΑ IB m2 m1 m3 Ic m4 (a) IAarrow_forwardConsider the circuit shown in the figure below. (Assume L = 5.20 m and R2 = 440 Ω.) (a) When the switch is in position a, for what value of R1 will the circuit have a time constant of 15.4 µs? (b) What is the current in the inductor at the instant the switch is thrown to position b?arrow_forwardCan someone helparrow_forwardCan someone help mearrow_forwardA particle in a box between x=0 and x=6 has the wavefunction Psi(x)=A sin(2πx). How muchenergy is required for the electron to make a transition to Psi(x)= A’ sin(7π x/3). Draw anapproximate graph for the wavefunction. Find A and A'arrow_forwardA proton is moving with 10^8 m/s speed. Find the De Broglie wavelength associated with theproton and the frequency of that wave.arrow_forwardFind the wavelength of the photon if a (Li--) electron makes a transition from n=4 to n=3. Findthe Bohr radius for each state.arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

University Physics Volume 3
Physics
ISBN:9781938168185
Author:William Moebs, Jeff Sanny
Publisher:OpenStax


Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning