
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31, Problem 31.27P
Draw the structure of the
a. 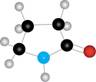 b.
b. 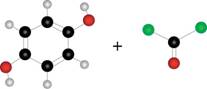
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
please help by drawing the mechanism with arrows of this reaction please
Name the following molecules using iupac
Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophen
Chapter 31 Solutions
Organic Chemistry
Ch. 31 - Prob. 31.1PCh. 31 - Prob. 31.2PCh. 31 - Prob. 31.3PCh. 31 - Draw the mechanism for the radical polymerization...Ch. 31 - Prob. 31.5PCh. 31 - Prob. 31.6PCh. 31 - Prob. 31.7PCh. 31 - Prob. 31.8PCh. 31 - Prob. 31.9PCh. 31 - Prob. 31.10P
Ch. 31 - Prob. 31.11PCh. 31 - Problem 30.12
What polymer is formed by anionic...Ch. 31 - Prob. 31.13PCh. 31 - Prob. 31.14PCh. 31 - Problem 30.15
What polyamide is formed from each...Ch. 31 - Prob. 31.16PCh. 31 - Prob. 31.17PCh. 31 - Prob. 31.18PCh. 31 - Prob. 31.19PCh. 31 - Prob. 31.20PCh. 31 - Prob. 31.21PCh. 31 - Prob. 31.22PCh. 31 - Prob. 31.23PCh. 31 - Prob. 31.24PCh. 31 - Prob. 31.25PCh. 31 - Prob. 31.26PCh. 31 - 30.26 Draw the structure of the polymer formed by...Ch. 31 - Prob. 31.28PCh. 31 - Prob. 31.29PCh. 31 - Prob. 31.30PCh. 31 - Prob. 31.31PCh. 31 - Prob. 31.32PCh. 31 - Prob. 31.33PCh. 31 - Prob. 31.34PCh. 31 - Prob. 31.35PCh. 31 - Prob. 31.36PCh. 31 - Prob. 31.37PCh. 31 - Prob. 31.38PCh. 31 - Prob. 31.39PCh. 31 - 30.39 Draw a stepwise mechanism for the...Ch. 31 - 30.40 Cationic polymerization of 3-phenylpropene ...Ch. 31 - Prob. 31.42PCh. 31 - Prob. 31.43PCh. 31 - 30.43 Although styrene undergoes both cationic and...Ch. 31 - 30.44 Rank the following compounds in order of...Ch. 31 - Prob. 31.46PCh. 31 - Prob. 31.47PCh. 31 - 30.47 Draw a stepwise mechanism for the following...Ch. 31 - 30.48 Draw a stepwise mechanism for the reaction...Ch. 31 - Prob. 31.50PCh. 31 - Prob. 31.51PCh. 31 - Prob. 31.52PCh. 31 - 30.52 (a) Explain why poly (vinyl alcohol) cannot...Ch. 31 - Prob. 31.54PCh. 31 - 30.53 Devise a synthesis of terephthalic acid and...Ch. 31 - Prob. 31.56PCh. 31 - Prob. 31.57PCh. 31 - 30.56 Compound A is a novel poly (ester amide)...Ch. 31 - Researchers at Rutgers University have developed...Ch. 31 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 31 - 30.59 Although chain branching in radical...Ch. 31 - Prob. 31.62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
- Which of these compounds is Ester formed from the reaction of acetic acid and one propanol arrow_forwardDescribe the four labeled parts of the reaction diagram from the reaction of sucrose, breaking down with and without an enzyme.arrow_forwardHow to determine the product with mechanism showedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY