
Interpretation:
The meaning of electroosmotic flow and the reason for itsoccurrence needs to be explained.
Concept introduction:
When electric field is applied in a liquid, the ions present in the liquid moves under the influence of potential gradient. This flow of liquid is termed as electroosmotic flow. It is most obvious is those having small channels. It occurs in buffered solutions and natural water.
Answer to Problem 30.1QAP
Electroosmotic flow of liquid is caused by gradient in potential across the capillary tube on the flowing liquid that has dissolved ions. This is caused when the capillary walls get electrically charged.
Explanation of Solution
When a capillary tube is filled with fluid having dissolved ions, and electrical field is applied, the buffer ions move to respond to the electrical charges. As the solvent which is water is neutral, it remains stationary and the fluid ions move towards its electrodes with respect to the charges applied. Below is depiction of movement of electrons:
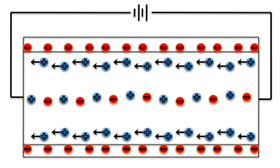
There is coulombic force induced by charges of ions in the solution wherein its net mobile charge causes electroosmotic flow. During this process of maintaining
Electroosmotic flow of liquid is caused due to a potential gradient across the capillary tube on the flowing liquid containing dissolved ions. This is caused when the capillary walls get electrically charged due to the application of electric field from outside.
Want to see more full solutions like this?
Chapter 30 Solutions
Principles of Instrumental Analysis, 6th Edition
- K Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

