
Concept explainers
Complete the following table by writing formulas for the compounds formed when the cations listed across the top combine with the anions listed along the side.
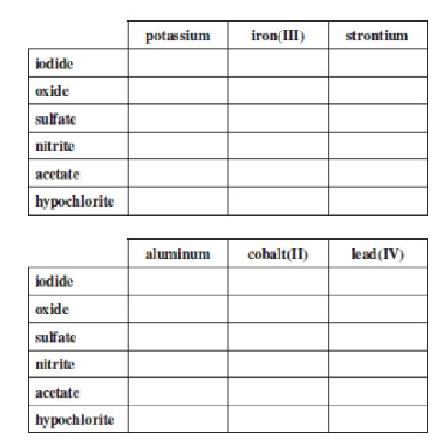
Interpretation:
The given cations combine with anions to form chemical formulas are to be analyzed.
Concept Introduction:
An ionic compound consists of metal cation and a non-metal anion. The rules that are used for determining the chemical formula of ionic compounds are as follows:
First, identify the symbol of the elements in the periodic table.
Identify the charges on the cation and the anion.
After that, the symbol of the cation is written followed by that of the anion.
It is very important that the compound is electrically neutral or the charges of anions and cations are neutralized in the compound.
Explanation of Solution
Given Information:Table is provided in the question.
Potassium cation
The potassium, iron(III), strontium, aluminum, cobalt(II), and lead(IV) cations are combined with iodide, oxide, sulfate, nitrite, acetate, and hypochlorite anions to form chemical formulae of ionic compounds that are shown in the above table.
Want to see more full solutions like this?
Chapter 3 Solutions
Introduction to Chemistry
- Q2: Label the following molecules as chiral or achiral, and label each stereocenter as R or S. CI CH3 CH3 NH2 C CH3 CH3 Br CH3 X &p Bra 'CH 3 "CH3 X Br CH3 Me - N OMe O DuckDuckarrow_forward1. For the four structures provided, Please answer the following questions in the table below. a. Please draw π molecular orbital diagram (use the polygon-and-circle method if appropriate) and fill electrons in each molecular orbital b. Please indicate the number of π electrons c. Please indicate if each molecule provided is anti-aromatic, aromatic, or non- aromatic TT MO diagram Number of π e- Aromaticity Evaluation (X choose one) Non-aromatic Aromatic Anti-aromatic || ||| + IVarrow_forward1.3 grams of pottasium iodide is placed in 100 mL of o.11 mol/L lead nitrate solution. At room temperature, lead iodide has a Ksp of 4.4x10^-9. How many moles of precipitate will form?arrow_forward
- Q1: Draw the most stable and the least stable Newman projections about the C2-C3 bond for each of the following isomers (A-C). Are the barriers to rotation identical for enantiomers A and B? How about the diastereomers (A versus C or B versus C)? H Br H Br (S) CH3 (R) CH3 H3C (S) H3C H Br Br H A C enantiomers H Br H Br (R) CH3 H3C (R) (S) CH3 H3C H Br Br H B D identicalarrow_forward2. Histamine (below structure) is a signal molecule involved in immune response and is a neurotransmitter. Histamine features imidazole ring which is an aromatic heterocycle. Please answer the following questions regarding Histamine. b a HN =N C NH2 a. Determine hybridization of each N atom (s, p, sp, sp², sp³, etc.) in histamine N-a hybridization: N-b hybridization: N-c hybridization: b. Determine what atomic orbitals (s, p, sp, sp², sp³, etc.) of the lone pair of each N atom resided in N-a hybridization: N-b hybridization: N-c hybridization:arrow_forwardNonearrow_forward
- 29. Use frontier orbital analysis (HOMO-LUMO interactions) to decide whether the following dimerization is 1) thermally allowed or forbidden and 2) photochemically allowed or forbidden. +arrow_forward30.0 mL of 0.10 mol/L iron sulfate and 20.0 mL of 0.05 mol/L of silver nitrate solutions are mixed together. Justify if any precipitate would formarrow_forwardDoes the carbonyl group first react with the ethylene glycol, in an intermolecular reaction, or with the end alcohol, in an intramolecular reaction, to form a hemiacetal? Why does it react with the alcohol it does first rather than the other one? Please do not use an AI answer.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





