
LL ORG CHEM
6th Edition
ISBN: 9781264840083
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 51P
Predict the solubility of each of the following vitamins in
a. 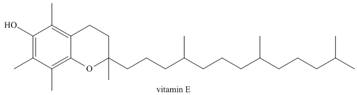 b.
b. 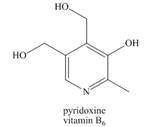
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.
Write the amididation reaction mechanism of p-aminophenol and acetic acid to produce acetaminophen please use arrows.
Name the following using IUPAC.
Chapter 3 Solutions
LL ORG CHEM
Ch. 3.1 - Prob. 1PCh. 3.2 - (a) Classify the carbon atoms in each compound as...Ch. 3.2 - Problem 3.3 Classify a carbon atom by the number...Ch. 3.2 - Classify each alkyl halide and alcohol as , or...Ch. 3.2 - Prob. 5PCh. 3.2 - Prob. 6PCh. 3.2 - Draw the structure of a compound of molecular...Ch. 3.2 - Prob. 8PCh. 3.2 - Prob. 9PCh. 3.2 - Draw the structure of a compound fitting each...
Ch. 3.4 - Predict which compound in each pair has the higher...Ch. 3.4 - Prob. 17PCh. 3.4 - a Label the hydrophobic and hydrophilic portions...Ch. 3.5 - Prob. 21PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 32PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 45PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 47PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 52PCh. 3 - Prob. 53PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 65PCh. 3 - Prob. 66PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forwardHow to draw the reaction mechasnism belowarrow_forwardName the following molecules with IUpacarrow_forward
- What is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forward
- Please help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forwardProvide solutionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY