
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + OWLv2, 1 term (6 months) Printed Access Card
6th Edition
ISBN: 9781305717367
Author: Mark S. Cracolice, Ed Peters
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 51E
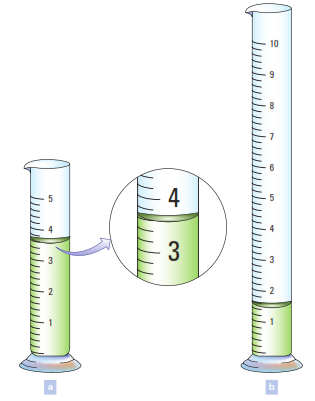
State the volume of liquid in each graduated cylinder in the figure below and explain how you decided upon the appropriate number of significant figures. The markings are calibrated to be read at the bottom of the curved surface, and the numbers represent milliliters.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1) How many monochlorination products-including stereochemistry- are there for the molecule below:
Select an amino acid that has and N-H or O-H bond in its R-group (you have 8 to choose from!). Draw at least two water molecules interacting with the R-group of the amino acid.
Is this aromatic?
Chapter 3 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + OWLv2, 1 term (6 months) Printed Access Card
Ch. 3 - Equivalency, conversion factor, solving...Ch. 3 - Metric system, SI units, derived unit, base unitCh. 3 - Prob. 3CLECh. 3 - Prob. 4CLECh. 3 - Prob. 5CLECh. 3 - Write the following numbers in scientific...Ch. 3 - Prob. 2ECh. 3 - Write the following numbers in ordinary decimal...Ch. 3 - Write the ordinary form of the following numbers:...Ch. 3 - Prob. 5E
Ch. 3 - Prob. 6ECh. 3 - Prob. 7ECh. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Prob. 10ECh. 3 - Complete the following operations:...Ch. 3 - Prob. 12ECh. 3 - What is the mathematical criterion for two...Ch. 3 - Sixty seconds and minute are equivalent...Ch. 3 - Write the equivalency for each conversion...Ch. 3 - Write the equivalency for each conversion...Ch. 3 - Write the equivalency and both conversion factors...Ch. 3 - Write the equivalency and both conversion factors...Ch. 3 - Prob. 19ECh. 3 - Prob. 20ECh. 3 - How long will it take to travel the 406 miles...Ch. 3 - A student who is driving home for the holidays...Ch. 3 - How many minutes does it take a car traveling 88...Ch. 3 - How many days are in 89 weeks?Ch. 3 - What will be the cost in dollars for nails for a...Ch. 3 - A student working for Stop and Shop is packing...Ch. 3 - An American tourist in Mexico was startled to see...Ch. 3 - How many nickels should you receive in exchange...Ch. 3 - How many weeks are in a decade?Ch. 3 - How many seconds are in the month of January?Ch. 3 - List at least two measurements that would be...Ch. 3 - List at least two measurements that would be made...Ch. 3 - A woman stands on a scale in an elevator in a tall...Ch. 3 - A person can pick up a large rock that is...Ch. 3 - What is the metric unit of length?Ch. 3 - What is the metric unit of mass?Ch. 3 - Kilo buck is a slang expression for a sum of...Ch. 3 - What is the difference between the terms kilo unit...Ch. 3 - One milliliter is equal to how many liters?Ch. 3 - How many centimeters are in a meter?Ch. 3 - Which unit, megagrams or grams, would be more...Ch. 3 - What is the name of the unit whose symbol is nm?...Ch. 3 - a 5.74 cg to g, b 1.41 kg to g, c 4.54 x 108 cg to...Ch. 3 - a 15.3 kg to g and mg, b 80.5 g to kg and mg, c...Ch. 3 - a 21.7 m to cm, b 517 m to km, c 0.666 km to cmCh. 3 - a 90.4 mm to m and cm, b 11.9 m to mm and cm, c...Ch. 3 - a 494 cm3 to mL, b 1.91 L to mL, c 874 cm3 to LCh. 3 - a 90.8 mL to L and cm3, b 16.9 L to mL and cm3, c...Ch. 3 - a 7.11 hg to g, b 5.27 x 107 m to pm, c 3.63 x 106...Ch. 3 - a 0.194 Gg to g, b 5.66 nm to m, c 0.00481 Mm to...Ch. 3 - State the volume of liquid in each graduated...Ch. 3 - How long is the object measured with the rulers...Ch. 3 - The same volume of liquid is in each measuring...Ch. 3 - Why is the length of the line in the illustration...Ch. 3 - Prob. 55ECh. 3 - Prob. 56ECh. 3 - Prob. 57ECh. 3 - a 52.20 mL helium, b 17.963 g nitrogen, c 78.45 mg...Ch. 3 - A moving-van crew picks up the following items: a...Ch. 3 - A solution is prepared by dissolving 2.86 grams of...Ch. 3 - Prob. 61ECh. 3 - An empty beaker has a mass of 94.33 grams. After...Ch. 3 - The mole is the SI unit for the amount of a...Ch. 3 - Exactly 1 liter of a solution contains 31.4 grams...Ch. 3 - An empty beaker with a mass of 42.3 g is filled...Ch. 3 - Use the definition density ; mass 4 volume to...Ch. 3 - 0.0715 gal = _____ c m 3 2.27 x 10 4 mL = _____ g...Ch. 3 - 19.3 L = _____ gal 0 .461 qt = _____ LCh. 3 - A popular breakfast cereal comes in a box...Ch. 3 - A copy of your chemistry textbook is found to have...Ch. 3 - The payload of a small pickup truck is 1450 pounds...Ch. 3 - The Hope diamond is the worlds largest blue...Ch. 3 - There is 115 mg of calcium in a 100-g serving of...Ch. 3 - The largest recorded difference of weight between...Ch. 3 - An Austrian boxer reads 69.1 kg when he steps on a...Ch. 3 - A woman gives birth to a 7.5-lb baby. How would a...Ch. 3 - The height of Angel Falls in Venezuela is 979 m....Ch. 3 - A penny is found to have a length of 1.97...Ch. 3 - The Willis Tower in Chicago is 1451 feet tall. How...Ch. 3 - What is the length of the Mississippi River in...Ch. 3 - The summit of Mount Everest is 29, 029 ft above...Ch. 3 - One of the smallest brilliant-cut diamonds ever...Ch. 3 - An office building is heated by oil-fired burners...Ch. 3 - A gas can is found to have a volume of 9.10...Ch. 3 - Celsius Fahrenheit Kelvin 69 -29 111 36 358 -141Ch. 3 - Celsius Fahrenheit Kelvin 40 590 -13 229 440 -314Ch. 3 - Normal body temperature is 98.6 F. What is this...Ch. 3 - In the winter, a heated home in the Northeast...Ch. 3 - Energy conservationists suggest that air...Ch. 3 - The milting point of an unknown solid is...Ch. 3 - The worlds highest shade temperature was recorded...Ch. 3 - The boiling point of a liquid is calculated to be...Ch. 3 - Prob. 93ECh. 3 - Prob. 94ECh. 3 - Prob. 95ECh. 3 - Prob. 96ECh. 3 - Prob. 97ECh. 3 - Prob. 98ECh. 3 - Prob. 99ECh. 3 - Prob. 100ECh. 3 - Prob. 101ECh. 3 - Rank the substances in the photograph from least...Ch. 3 - Calculate the density of benzene, a liquid used in...Ch. 3 - A general chemistry student found a chunk of metal...Ch. 3 - Densities of gases are usually measured in grams...Ch. 3 - Prob. 106ECh. 3 - Ether, a well-known anesthetic, has a density of...Ch. 3 - Prob. 108ECh. 3 - Determine the mass of 2.0 L rubbing alcohol, which...Ch. 3 - Calculate the volume occupied by 15.4 grams of...Ch. 3 - The mass of the liquid in the graduated cylinder...Ch. 3 - Prob. 112ECh. 3 - Distinguish precisely and in scientific terms the...Ch. 3 - Determine whether each statement that follows is...Ch. 3 - How tall are you in a meters; b decimeters; c...Ch. 3 - What do you weigh in a milligrams; b grams; c...Ch. 3 - Standard printer and copier paper is the United...Ch. 3 - The density of aluminium is 2.7 g/cm3. An ecology...Ch. 3 - What is the average density of a single marble...Ch. 3 - A woman has just given birth to a bouncing 6 lb, 7...Ch. 3 - A students drivers license lists her height as 5...Ch. 3 - How many grams of milk are in a 12.0 fluid-ounce...Ch. 3 - The fuel tank in an automobile has a capacity of...Ch. 3 - A welcome rainfall caused the temperature to drop...Ch. 3 - At high noon on the lunar equator the temperature...Ch. 3 - A recipe calls for a quarter cup of butter....Ch. 3 - Prob. 127ECh. 3 - In Active Example 3-29 you calculated that you...Ch. 3 - Write each of the following in scientific...Ch. 3 - Write each of the following numbers in ordinary...Ch. 3 - Prob. 3PECh. 3 - Prob. 4PECh. 3 - How many seconds are in 12 minutes?Ch. 3 - Prob. 6PECh. 3 - Prob. 7PECh. 3 - Prob. 8PECh. 3 - Prob. 9PECh. 3 - Prob. 10PECh. 3 - Prob. 11PECh. 3 - Prob. 12PECh. 3 - What is the volume of liquid in the buret in the...Ch. 3 - Prob. 14PECh. 3 - Round off each of the following quantities to two...Ch. 3 - Prob. 16PECh. 3 - At a certain temperature, 0.878 g of a pure liquid...Ch. 3 - Prob. 18PECh. 3 - Prob. 19PECh. 3 - Prob. 20PECh. 3 - Prob. 21PECh. 3 - Prob. 22PECh. 3 - Prob. 23PECh. 3 - Prob. 24PECh. 3 - The mass of a 50.00-milliliter sample of methanol...Ch. 3 - Prob. 26PECh. 3 - Determine the mass in kilograms of a lead brick...Ch. 3 - Prob. 28PECh. 3 - Prob. 29PE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CHEM2323 E Tt PS CH03 Draw and name all monobromo derivatives of pentane, C5H11Br. Problem 3-33 Name: Draw structures for the following: (a) 2-Methylheptane (d) 2,4,4-Trimethylheptane Problem 3-35 (b) 4-Ethyl-2,2-dimethylhexane (e) 3,3-Diethyl-2,5-dimethylnonane (c) 4-Ethyl-3,4-dimethyloctane 2 (f) 4-Isopropyl-3-methylheptane KNIE>arrow_forwardProblem 3-42 Consider 2-methylbutane (isopentane). Sighting along the C2-C3 bond: (a) Draw a Newman projection of the most stable conformation. (b) Draw a Newman projection of the least stable conformation. Problem 3-44 Construct a qualitative potential-energy diagram for rotation about the C-C bond of 1,2-dibromoethane. Which conformation would you expect to be most stable? Label the anti and gauche conformations of 1,2- dibromoethane. Problem 3-45 Which conformation of 1,2-dibromoethane (Problem 3-44) would you expect to have the largest dipole moment? The observed dipole moment of 1,2-dibromoethane is µ = 1.0 D. What does this tell you about the actual conformation of the molecule?arrow_forwardGas Law Studies 1. Mass of zinc Determination of 0.899 2) Moles of zinc 0.01361 mol 3.) Moles of hydrogen 00? ← I was told to calculate this number from mole of zinc. 350m So does that mean it will be 0.01361 mol too? 4 Volume of water collected (mL) 5) VL of water collected (Liters) 0.350 L 6) Temp of water collected (°C) 7) Temp of water collected (°K) 8) Atmospheric pressure (mm) 9) Vapor pressure of water (mm) 10) Corrected pressure of hydrogen 20% 29°C 764.0mm Hg (mm) 17.5mm 11) Corrected pressure of hydrogen (atm) 12) Experimentally calculated value of 19 13. Literature value of R 14) % Error 15) Suggest reasons for the % error (#14)arrow_forward
- No wedge or dashes. Do proper structure. Provide steps and explanation.arrow_forward10 Question (1 point) Draw curved arrow notation to indicate the proton transfer between NaOH and CH3CO₂H. 2nd attempt :0- H See Periodic Table See Hint Draw the products of the proton transfer reaction. Don't add a + sign between the products.arrow_forwardProvide steps and explanation please.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY