
Interpretation:
The leaving group in the given molecule is to be determined in order identify the positively charged and neutral fragments produced during mechanism.
Concept Introduction:
Heterolytic cleavage of sigma bond occurs under a variety of conditions. The mechanism of bond cleavage is as shown below,
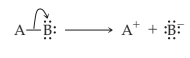
The arrow in the above mechanism indicates that the sigma electrons form A-B bond are leaving A and becoming the exclusive property of B. Since the fragment A is formally losing one electron, it must become positively charged and B must become negatively charged since it gains an electron.
Often, heterolytic cleavage occurs from a charged intermediate that was formed in a previous step. The process is the same, but the charge on the products is different.

Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
EBK PUSHING ELECTRONS
- The following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forwardThe chemical formula of "benzimidazole E" is C7H6N2. Draw it.arrow_forwardBriefly comment (without formulas) on the steps of the aldol condensation mechanism in acidic and basic media.arrow_forward
- The following intermediates are to proceed by acetoacetic ester synthesis. What are intermediates 1 and 2 plus the final product 3? Please include a detailed explanation and drawings of the intermediates and how they occurred.arrow_forwardWhat are the missing intermediates 1, 2, and 3? Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediates and how they occur.arrow_forwardWhat is the reactant that makes the following product of the reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forward
- Draw the products formed when each ester is hydrolyzed with water and sulfuric acid.arrow_forwardDraw the complete structural formula from each condensed structure. include all hydrogen atoms.arrow_forwardDraw the complete structural formula from each condensed structure. Include all hydrogen atoms.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





