
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 43P
In each of the following groups of compounds, identify the one with the largest heat of combustion and the one with the smallest. In which cases can a comparison of heats of combustion be used to assess relative stability?
Cyclopropane, cyclobutane, cyclopentane
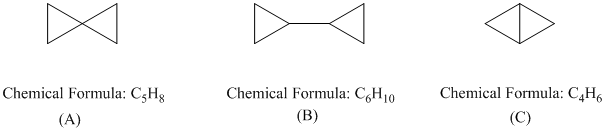
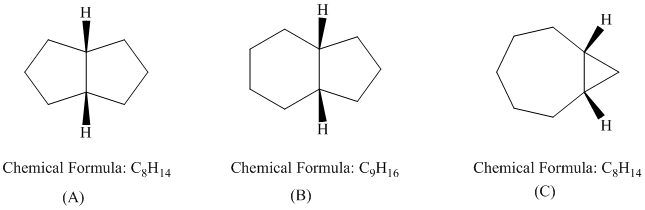
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.
Draw mechanism
Does Avogadro's number have units?
Chapter 3 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 3.1 - Identify the alkanes corresponding to each of the...Ch. 3.1 - Find the conformations in Figure 3.4 in which the...Ch. 3.2 - Sketch a potential energy diagram for rotation...Ch. 3.2 - Acetylcholine is a neurotransmitter in the central...Ch. 3.2 - Prob. 5PCh. 3.5 - The heats of combustion of ethylcyclopropane and...Ch. 3.8 - Prob. 7PCh. 3.10 - The following questions relate to a cyclohexane...Ch. 3.10 - Draw the most stable conformation of...Ch. 3.11 - Prob. 10P
Ch. 3.11 - Prob. 11PCh. 3.12 - Based on what you know about disubstituted...Ch. 3.12 - Write structural formulas for the most stable...Ch. 3.14 - Cubane (C4H8) is the common name of the polycyclic...Ch. 3.14 - Prob. 15PCh. 3.14 - Prob. 16PCh. 3.14 - Prob. 17PCh. 3.14 - Prob. 18PCh. 3.15 - Prob. 19PCh. 3 - Give the IUPAC names of each of the following: (a)...Ch. 3 - Draw Newman projections for the gauche and...Ch. 3 - Identify all atoms that are (a) anti and (b)...Ch. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Oxidation of 4-tert-butylthiane proceeds according...Ch. 3 - The following are representations of two forms of...Ch. 3 - Draw (a) a Newman projection of the most stable...Ch. 3 - Write a structural formula for the most stable...Ch. 3 - Sight down the C-2-C-3 bond, and draw Newman...Ch. 3 - Prob. 34PCh. 3 - Sketch an approximate potential energy diagram for...Ch. 3 - Prob. 36PCh. 3 - Even though the methyl group occupies an...Ch. 3 - Which do you expect to be the more stable...Ch. 3 - Arrange the trimethylcyclohexane isomers shown in...Ch. 3 - Identify the more stable stereoisomer in each of...Ch. 3 - One stereoisomer of 1,1,3,5-tetramethylcyclohexane...Ch. 3 - One of the following two stereoisomers is...Ch. 3 - In each of the following groups of compounds,...Ch. 3 - The heats of combustion of the more and less...Ch. 3 - The measured dipole moment of ClCH2CH2Cl is 1.12D....Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48DSPCh. 3 - Prob. 49DSPCh. 3 - Prob. 50DSPCh. 3 - Prob. 51DSPCh. 3 - Prob. 52DSPCh. 3 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License