
(a)
Interpretation:
The element has to be identified from the total number of electrons present that has electronic configuration as
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
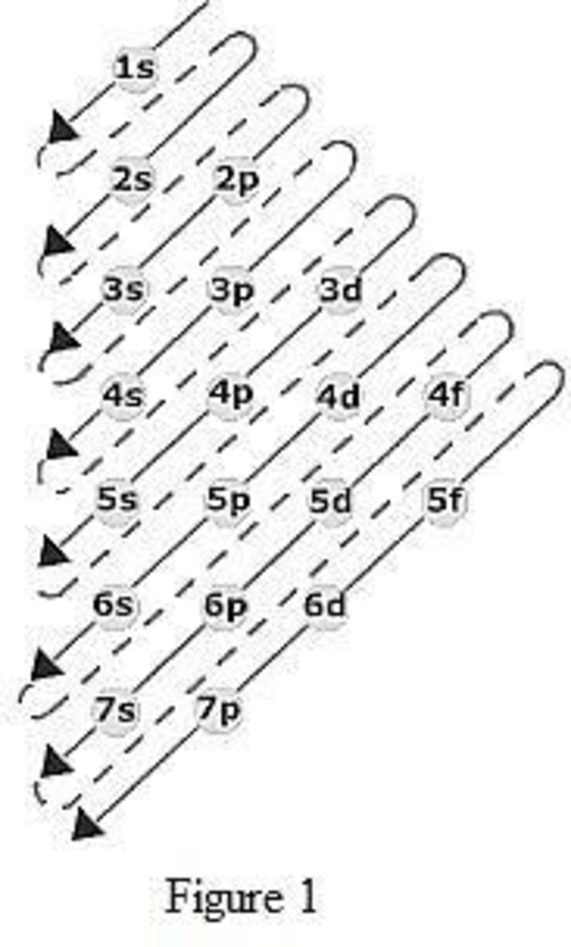
(b)
Interpretation:
The element has to be identified from the total number of electrons present that has electronic configuration as
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
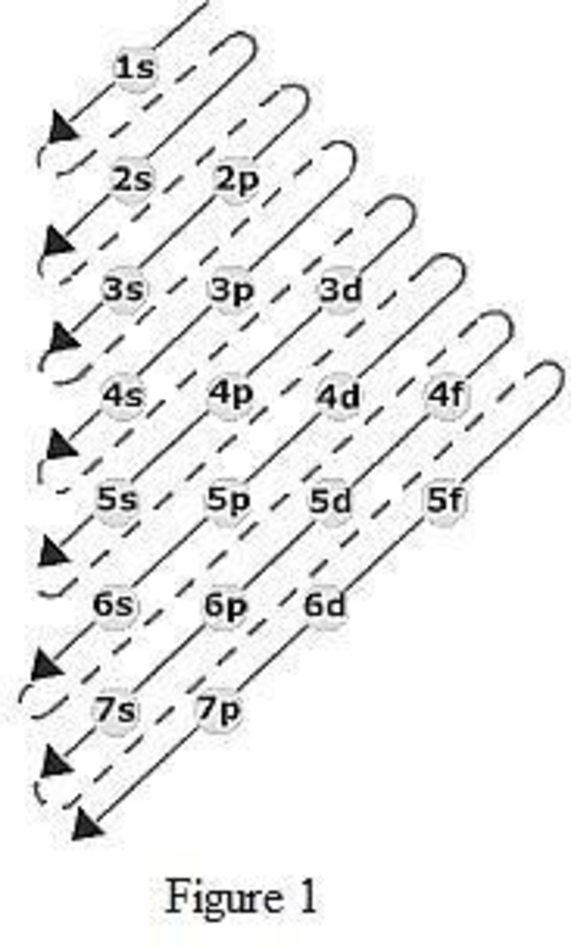
(c)
Interpretation:
The element has to be identified from the total number of electrons present that has electronic configuration as
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
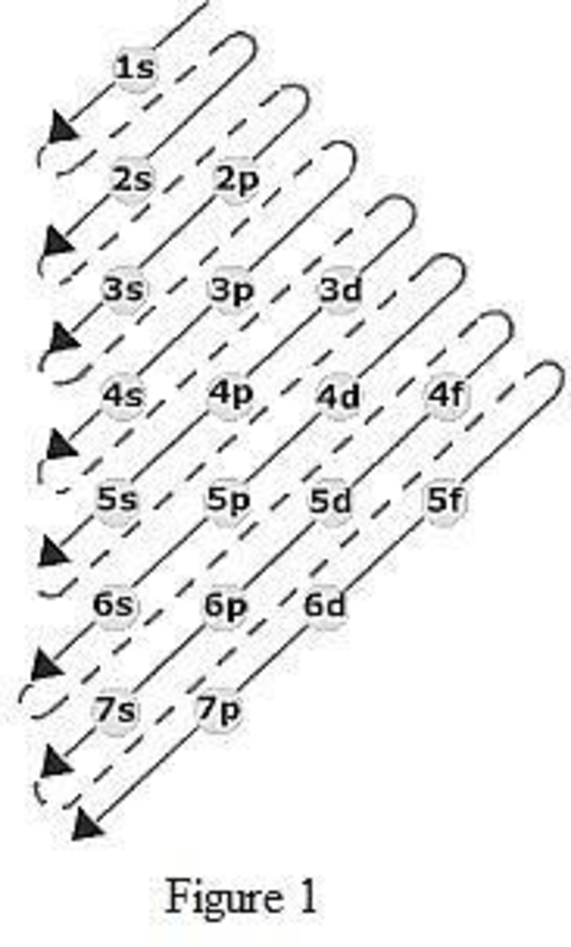
(d)
Interpretation:
The element has to be identified from the total number of electrons present that has electronic configuration as
Concept Introduction:
Electronic configuration of an atom describes how many electrons are present in the shell. Many orbitals are present about the nucleus of an atom. In these orbitals the electrons do not occupy randomly. There are three rules for assigning the electrons to various shells, subshells, and orbitals. They are,
- The subshells are filled in increasing order of energy.
- In a subshell, the electrons occupy the orbital singly first in all orbitals before pairing up by the second electron. All the electrons that are in singly occupied orbitals have same spin.
- In a given orbital there cannot be more than two electrons and they have opposite spins.
Electronic configuration of an element is the one that gives information about how many electrons are present in each electron subshell of an atom. The electrons are added to the subshells in increasing order of energy. Electronic configurations are written in shorthand notation which uses a number‑letter combination. The shell is indicated by the number and subshell is indicated by the letter. Superscript that follows the subshell tells how many electrons are present in the subshell.
The order of filling up the electrons in the subshell is done as shown in the given figure below.
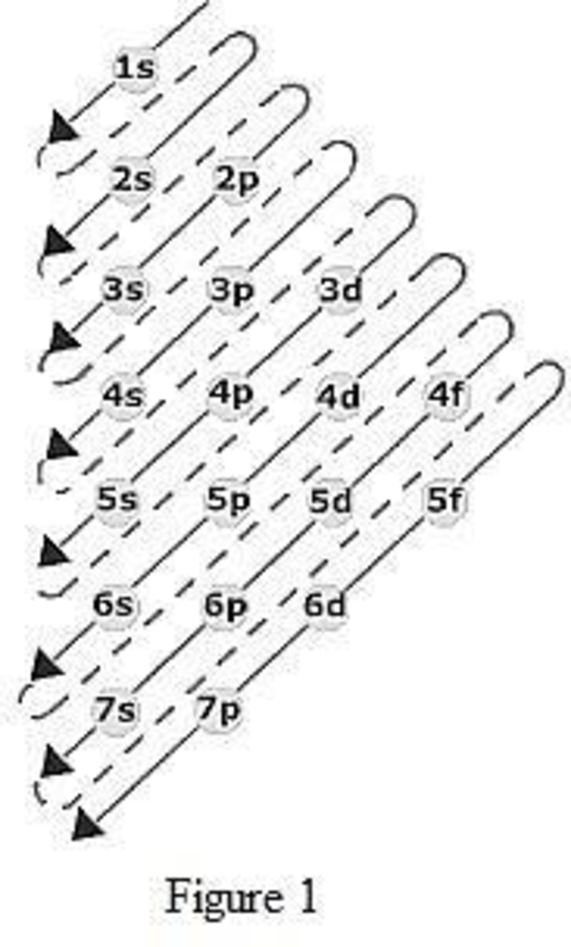
Trending nowThis is a popular solution!

Chapter 3 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Indicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forwardIndicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are mixed with sodium ethoxide in ethanol.arrow_forward
- Indicate the products obtained if 2,2-dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol.arrow_forward2,2-Dimethylpropanal and acetaldehyde are reacted with sodium ethoxide in ethanol. Indicate the products obtained.arrow_forwardAdd conditions above and below the arrow that turn the reactant below into the product below in a single transformationADS fint anditions 百 Abl res condinese NC ง Add on condtions 1.0 B H,N.arrow_forward
- Steps on how to solve. Thank you!arrow_forward3. Name this ether correctly. H₁C H3C CH3 CH3 4. Show the best way to make the ether in #3 by a Williamson Ether Synthesis. Start from an alcohol or phenol. 5. Draw the structure of an example of a sulfide.arrow_forward1. Which one(s) of these can be oxidized with CrO3 ? (could be more than one) a) triphenylmethanol b) 2-pentanol c) Ethyl alcohol d) CH3 2. Write in all the product(s) of this reaction. Label them as "major" or "minor". 2-methyl-2-hexanol H2SO4, heatarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





