
CHEMISTRY-TEXT
8th Edition
ISBN: 9780134856230
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3.32CP
The following diagram represents the reaction of
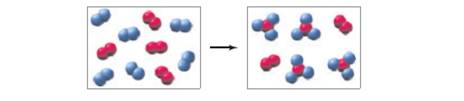
(a) Write a balanced equation for the reaction, and identify the limiting reactant.
(b) How many moles of product can he made from 1.0 moIof
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in
your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on
the LC-MS printout. How much different are they?
2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit,
explain what each of these is and why they are present.
3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by
calculating the accurate monoisotopic mass.
4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum
of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source.
5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one
point of extra credit, see if you can identify this molecule as well and confirm by…
Please draw, not just describe!
can you draw each step on a piece of a paper please this is very confusing to me
Chapter 3 Solutions
CHEMISTRY-TEXT
Ch. 3 - Prob. 3.1PCh. 3 - If blue spheres represent nitrogen atoms and red...Ch. 3 - Prob. 3.3PCh. 3 - APPLY 3.4 The major ingredient in ordinary safety...Ch. 3 - Calculate the molecular weight of sulfuric acid...Ch. 3 - Conceptual APPLY 3.6 Use the structural formula of...Ch. 3 - PRACTICE 3.7 How many moles arc in 5.26 g of...Ch. 3 - APPLY 3.8 When a diabetic experiences low blood...Ch. 3 - PRACTICE 3.9 Aspirin is prepared by reaction...Ch. 3 - APPLY 3.10 Refer to the balanced reaction for the...
Ch. 3 - PRACTICE 3.11 Ethyl alcohol is prepared...Ch. 3 - APPLY 3.12 (a) Diethyl ether (C4H10O), the “ether”...Ch. 3 - Conceptual PRACTICE 3.13 The following diagram...Ch. 3 - Conceptual APPLY 3.14 Draw a diagram similar to...Ch. 3 - Lithium oxide is used aboard the space shuttle to...Ch. 3 - APPLY 3.16 After lithium hydroxide is produced...Ch. 3 - PRACTICE 3.17 What is the empirical formula of the...Ch. 3 - Conceptual APPLY 3.19 Use the structural formula...Ch. 3 - PRACTICE 3.20 Menthol, a flavouring agent obtained...Ch. 3 - PRACTICE 321 Combustion analysis is performed on...Ch. 3 - PRACTICE 3.23 A compound has an empirical formula...Ch. 3 - APPLY 3.24 Combustion analysis was performed on...Ch. 3 - Prob. 3.23PCh. 3 - Match the terms percent yield and percent atom...Ch. 3 - Examine two reactions important in chemical...Ch. 3 - Propene is a raw material for a wide variety of...Ch. 3 - Ibuprofen (the active ingredient in the...Ch. 3 - The original synthesis for ibuprofen, developed in...Ch. 3 - The reaction of A (red spheres) with B (blue...Ch. 3 - The diagrams represent a reaction on the molecular...Ch. 3 - Fluoxetine, marketed as an antidepressant under...Ch. 3 - The following diagram represents the reaction of...Ch. 3 - What is the percent composition of cysteine, one...Ch. 3 - Cytosine, a constituent of deoxyribonucleic acid...Ch. 3 - A hydrocarbon of unknown formula CxHy was...Ch. 3 - Which of the following equations are balanced? (a)...Ch. 3 - Which of the following equations are balanced?...Ch. 3 - Balance the following equations: (a)...Ch. 3 - Balance the following equations: (a) The explosion...Ch. 3 - Balance the following equations: (a)...Ch. 3 - Balance the following equations:...Ch. 3 - Balance the following equations. (a)...Ch. 3 - Balance the following equations. (a) CO(...Ch. 3 - What are the molecular (formula) weights of the...Ch. 3 - What are the formulas of the following substances?...Ch. 3 - What are the molecular weights of the following...Ch. 3 - Prob. 3.47SPCh. 3 - How many grams are in a mole of each of the...Ch. 3 - Prob. 3.49SPCh. 3 - How many moles of ions are in 27.5 g of MgCl2?Ch. 3 - How many moles of anions are in 35.6 g of AlF3?Ch. 3 - What is the molecular weight of chloroform if...Ch. 3 - What is the molecular weight of cholesterol if...Ch. 3 - 3.52 Iron (II) sulfate, FeSO4, is prescribed for...Ch. 3 - Prob. 3.55SPCh. 3 - An average cup of coffee contains about 125 mg of...Ch. 3 - Prob. 3.57SPCh. 3 - A sample that weighs 25.12 g contains 6.0221023...Ch. 3 - Prob. 3.59SPCh. 3 - Prob. 3.60SPCh. 3 - Prob. 3.61SPCh. 3 - In the preparation of iron from hematite, Fe2O3...Ch. 3 - An alternative method for preparing pure iron from...Ch. 3 - Magnesium metal burns in oxygen to form...Ch. 3 - Ethylene gas, C2H4 , reacts with water at high...Ch. 3 - Prob. 3.66SPCh. 3 - Titanium dioxide (TiO2) , the substance used as...Ch. 3 - Prob. 3.68SPCh. 3 - Aluminum reacts with oxygen to yield aluminum...Ch. 3 - The industrial production of hydriodic acid takes...Ch. 3 - An alternative method for producing hydriodic acid...Ch. 3 - Nickel(II) sulfate, used for nickel plating, is...Ch. 3 - Hydrazine, N2H4 , once used as a rocket...Ch. 3 - Assume that you have 1.39 mol of H2 and 3.44 mol...Ch. 3 - Hydrogen and chlorine react to yield hydrogen...Ch. 3 - How many grams of the dry-cleaning solvent...Ch. 3 - How many grams of each product result from the...Ch. 3 - Limestone (CaCO3) reacts with hydrochloric acid...Ch. 3 - Sodium azide (NaN3) yields N2 gas when heated to...Ch. 3 - Acetic acid (CH3CO2H) reacts with isopentyl...Ch. 3 - Cisplatin [Pt( NH 3)2Cl2] , a compound used in...Ch. 3 - If 1.87 g of acetic acid (CH3COOH) reacts with...Ch. 3 - If 3.42 g of K2PtCl4 and 1.61 g of NH3 give 2.08 g...Ch. 3 - The reaction of tungsten hexachloride (WCl6) with...Ch. 3 - Sodium borohydride, NaBH4 , a substance used in...Ch. 3 - Urea, a substance commonly used as a fertilizer,...Ch. 3 - Calculate the mass percent composition of each of...Ch. 3 - What are the empirical formulas of substances with...Ch. 3 - Ferrocene, a substance proposed for use as a...Ch. 3 - What is the empirical formula of stannous...Ch. 3 - What are the empirical formulas of each of the...Ch. 3 - An unknown liquid is composed of 5.57% 11, 28.01 %...Ch. 3 - An unknown liquid is composed of 34.31% C, 5.28%...Ch. 3 - Combustion analysis of 45.62 mg of toluene, a...Ch. 3 - Coniine, a toxic substance isolated from poison...Ch. 3 - Cytochrome c is an iron—containing enzyme found in...Ch. 3 - Nitrogen fixation in the root nodules of peas and...Ch. 3 - Disilane, Si2Hx, is analyzed and found to contain...Ch. 3 - A certain metal sulfide, MS2, is used extensively...Ch. 3 - Combustion analysis of a 31.472 mg sample of the...Ch. 3 - The stimulant amphetamine contains only carbon,...Ch. 3 - Prob. 3.102SPCh. 3 - Prob. 3.103SPCh. 3 - Prob. 3.104SPCh. 3 - Prob. 3.105SPCh. 3 - Prob. 3.106SPCh. 3 - Prob. 3.107SPCh. 3 - Prob. 3.108MPCh. 3 - The molar mass of HCl is 36.5 g/mol, and the...Ch. 3 - Assume that gasoline has the formula C8H18 and has...Ch. 3 - Compound X contains only carbon, hydrogen,...Ch. 3 - Prob. 3.112MPCh. 3 - A certain alcoholic beverage contains only ethanol...Ch. 3 - A mixture of FeO and Fe2O3 with a mass of 10.0 g...Ch. 3 - Prob. 3.115MPCh. 3 - When eaten, dietary carbohydrates are digested to...Ch. 3 - Prob. 3.117MPCh. 3 - Prob. 3.118MPCh. 3 - A mixture of XCl3 and XCl5 weighing 10.00 g...Ch. 3 - Ammonium nitrate, a potential ingredient of...Ch. 3 - Window glass is typically made by mixing soda ash...Ch. 3 - Prob. 3.122MPCh. 3 - Ethylene glycol, commonly used as automobile...Ch. 3 - Prob. 3.124MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
- Predict the major products of this organic reaction: HBr (1 equiv) Δ ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. Explanation Check X ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacyarrow_forwardFor the structure below, draw the resonance structure that is indicated by the curved arrow(s). Be sure to include formal charges. :ÖH Modify the second structure given to draw the new resonance structure. Include lone pairs and charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds.arrow_forwardUsing the table of Reactants and Products provided in the Hints section, provide the major product (with the correct stereochemistry when applicable) for questions below by selecting the letter that corresponds to the exact chemical structures for the possible product. OH conc Hydrochloric acid 40°C Temp A/arrow_forward
- Using arrows to designate the flow of electrons, complete the reaction below and provide a detailed mechanism for the formation of the product OH conc Hydrochloric acid 40°C Temp All chemical structures should be hand drawn on a piece of paper Paragraph BI UAE +varrow_forwarddraw out the following structures plesearrow_forwardDraw everything on a piece of paper outlining the synthesis from acetaldehyde to 2 cyclopentene carboxaldehyde using carbon based reagants with 3 carbons or fewers. Here is the attached image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY