1 Chemical Tools Experimentation And Measurement 2 Atoms, Molecules, And Ions 3 Mass Relationships In Chemical Reactions 4 Reactions In Aqueous Solution 5 Periodicity And The Electronic Structure Of Atoms 6 Ionic Compounds Periodic Trends And Bonding Theory 7 Covalent Bonding And Electron-Dot Structures 8 Covalent Compounds Bonding Theories And Molecular Structure 9 Thermochemistry Chemical Energy 10 Gases Their Properties And Behavior 11 Liquids, Solids, And Phase Changes 12 Solutions And Their Properties 13 Chemical Kinetics 14 Chemical Equilibrium 15 Aqueous Equilibria Acids And Bases 16 Applications Of Aqueous Equilibria 17 Thermodynamica Entropy, Free Energy And Equilibrium 18 Electrochemistry 19 Nuclear Chemistry 20 Transition Elements And Coordination Chemistry 21 Metals And Solid-State Materials 22 The Main-Group Elements 23 Organic And Biological Chemistry expand_more
Chapter Questions expand_more
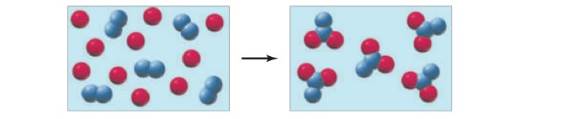
Problem 3.1P Problem 3.2A Problem 3.3P Problem 3.4A: APPLY 3.4 The major ingredient in ordinary safety matches is potassium chlorate, KClO3, a substance... Problem 3.5P Problem 3.6A: Conceptual APPLY 3.6 Use the structural formula of sucrose to determine its molecular weight and... Problem 3.7P: PRACTICE 3.7 How many moles arc in 5.26 g of NaHCO3, the main ingredient in Alka Seltzer tablets? Problem 3.8A: APPLY 3.8 When a diabetic experiences low blood glucose, possibly due to an excess of insulin or... Problem 3.9P: PRACTICE 3.9 Aspirin is prepared by reaction salicyclic acid (C7H6O3) with acetic anhydride (C4H6O3)... Problem 3.10A: APPLY 3.10 Refer to the balanced reaction for the synthesis aspirin in Problem 3.9. (a) How many... Problem 3.11P: PRACTICE 3.11 Ethyl alcohol is prepared industrially by the react ion of ethylene, C2H4, with water.... Problem 3.12A: APPLY 3.12 (a) Diethyl ether (C4H10O), the “ether” used medically as an aesthetic, is prepared... Problem 3.13P: Conceptual PRACTICE 3.13 The following diagram represents the reaction of A (red spheres) with B,... Problem 3.14A: Conceptual APPLY 3.14 Draw a diagram similar to the one shown in Problem 3.13 for the following... Problem 3.15P Problem 3.16A: APPLY 3.16 After lithium hydroxide is produced aboard the space shuttle by reaction of Li2O with H2O... Problem 3.17P: PRACTICE 3.17 What is the empirical formula of the ingredient in Bufferin tablets that has the... Problem 3.18P Problem 3.19A: Conceptual APPLY 3.19 Use the structural formula for glucose to determine the molecular formula.... Problem 3.20P: PRACTICE 3.20 Menthol, a flavouring agent obtained from peppermint oil, contains carbon, hydrogen,... Problem 3.21P: PRACTICE 321 Combustion analysis is performed on 0.50 g of a hydrocarbon, and 1.55 g of CO2 and... Problem 3.22A: APPLY 3.22 (a) Polychlorinated biphenyls (PCBs) were compounds used as coolants in transformers and... Problem 3.23P: PRACTICE 3.23 A compound has an empirical formula of C6H5 as determined from combustion analysis.... Problem 3.24A: APPLY 3.24 Combustion analysis was performed on 1.00 g of a compound containing C, H, and N, and... Problem 3.25P Problem 3.26P: PROBLEM 3.26 (a) Balance the reaction for photosynthesis. (b) If one acre of corn absorbs 1400 lb of... Problem 3.27P Problem 3.28P Problem 3.29CP: The reaction of A (red spheres) with B (blue spheres) is shown in the following diagram: Which... Problem 3.30CP Problem 3.31CP Problem 3.32CP Problem 3.33CP Problem 3.34CP Problem 3.35CP Problem 3.36SP: Which of the following equations are balanced? (a) The development reaction in silver-halide... Problem 3.37SP: Which of the following equations are balanced? Balance any that need it. (a) The thermite reaction,... Problem 3.38SP: Balance the following equations: (a) Mg+HNO3H2+Mg(NO3)2 (b) CaC2+H2OCa(OH)2+C2H2 (c) S+O2SO3 (d)... Problem 3.39SP: Balance the following equations: (a) The explosion of ammonium nitrate: NH4NO3N2+O2+H2O (b) The... Problem 3.40SP: Balance the following equations: (a) SiCl4+H2OSiO2+HCl (b) P4O10+H2OH3PO4 (c) CaCN2+H2OCaCO3+NH3 (d)... Problem 3.41SP: Balance the following equations: aVCl3+Na+COVCO6+NaClbRuI3+CO+AgRuCO5+AgIcCoS+CO+CuCo2CO8+Cu2S Problem 3.42SP: What are the molecular (formula) weights of the following substances? (a) Hg2Cl2 (calomel, used at... Problem 3.43SP: What are the formulas of the following substances? (a) PCl?; Mol. wt. = 137.3 (b) Nicotine,... Problem 3.44SP: What are the molecular weights of the following pharmaceuticals? (a) C33H35FN2O5 (atorvastatin,... Problem 3.45SP Problem 3.46SP: How many grams are in a mole of each of the following substances? (a) Ti (c) Hg (b) Br2 (d) H2O Problem 3.47SP Problem 3.48SP: How many moles of ions are in 27.5 g of MgCl2? Problem 3.49SP: How many moles of anions are in 35.6 g of AlF3? Problem 3.50SP: What is the molecular weight of chloroform if 0.0275mol weighs 3.28 g? Problem 3.51SP: What is the molecular weight of cholesterol if 0.5731mol weighs 221.6 g? Problem 3.52SP: 3.52 Iron (II) sulfate, FeSO4, is prescribed for the treatment of anemia. How many moles of FeSO4... Problem 3.53SP Problem 3.54SP: An average cup of coffee contains about 125 mg of caffeine, C8H10N4O2. How many moles of caffeine... Problem 3.55SP Problem 3.56SP: A sample that weighs 25.12 g contains 6.0221023 particles. If 25.00% of the total numbers of... Problem 3.57SP Problem 3.58SP Problem 3.59SP Problem 3.60SP Problem 3.61SP: An alternative method for preparing pure iron from Fe2O3 is by reaction with carbon monoxide:... Problem 3.62SP Problem 3.63SP Problem 3.64SP Problem 3.65SP Problem 3.66SP Problem 3.67SP Problem 3.68SP Problem 3.69SP Problem 3.70SP Problem 3.71SP: 3.71 Hydrogen and chlorine react to yield hydrogen chloride:
How many grams of HCl are formed from... Problem 3.72SP Problem 3.73SP Problem 3.74SP: Nickel (ll) sulfate, used for nickel plating, is prepared by treat- ment of nickel (ll) carbonate... Problem 3.75SP Problem 3.76SP Problem 3.77SP Problem 3.78SP Problem 3.79SP Problem 3.80SP Problem 3.81SP Problem 3.82SP Problem 3.83SP Problem 3.84SP Problem 3.85SP Problem 3.86SP Problem 3.87SP: What are the empirical formulas of each of the following substances? (a) Ibuprofen, a headache... Problem 3.88SP Problem 3.89SP: 3.89 Coniine, a toxic substance isolated from poison hemlock, contains only carbon, hydrogen, and... Problem 3.90SP Problem 3.91SP Problem 3.92SP Problem 3.93SP Problem 3.94SP Problem 3.95SP Problem 3.96SP Problem 3.97SP Problem 3.98SP: 3.98 The molecular weight of an organic compound was found to be 70.042 11 by mass spectrometry. Is... Problem 3.99SP Problem 3.100SP Problem 3.101SP: (a) Combustion analysis of 150.0 mg of 1, 2, 3, benzenetriol, a compound composed of carbon,... Problem 3.102CP Problem 3.103CP Problem 3.104CP Problem 3.105CP Problem 3.106CP Problem 3.107CP Problem 3.108CP Problem 3.109CP: Ferrocene, a substance proposed for use as a gasoline additive, has the percent composition 5.42% H,... Problem 3.110CP Problem 3.111CP: Ethylene glycol, commonly used as automobile antifreeze, contains only carbon, hydrogen, and oxygen.... Problem 3.112CP Problem 3.113CP Problem 3.114CP Problem 3.115CP Problem 3.116CP: A pulverized rock sample believed to be pure calcium carbonate, CaCO3, is subjected to chemical... Problem 3.117CP Problem 3.118CP Problem 3.119CP Problem 3.120CP Problem 3.121CP Problem 3.122CP Problem 3.123CP Problem 3.124CP Problem 3.125CP Problem 3.126CP format_list_bulleted


 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning




