
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
7th Edition
ISBN: 9780133900811
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3.101SP
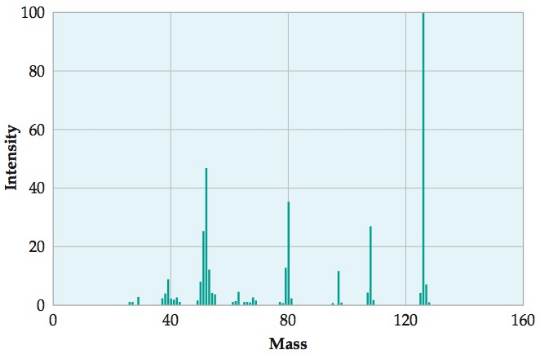
(a) Combustion analysis of 150.0 mg of 1, 2, 3, benzenetriol, a compound composed of carbon, hydrogen, and oxygen, gives 64.3 mg of H2O and 314.2 mg of CO2. What is the empirical formula of 1, 2, 3, benzenetriol?
(b) Given the mass spectrum of identify the molecular weight and give the molecular formula.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Calculating the pH of a weak base titrated with a strong acid
b
An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37.
Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added.
Round your answer to 2 decimal places.
pH = ☐
☑
5
QUESTION: Find the standard deviation for the 4 different groups
5.298
3.977
223.4
148.7
5.38
4.24
353.7
278.2
5.033
4.044
334.6
268.7
4.706
3.621
305.6
234.4
4.816
3.728
340.0
262.7
4.828
4.496
304.3
283.2
4.993
3.865
244.7
143.6
STDEV =
STDEV =
STDEV =
STDEV =
QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression'
*The images of the data showing 'coefficients for the standard curve' have been provided
Chapter 3 Solutions
Chemistry, Books a la Carte Plus Mastering Chemistry with eText -- Access Card Package (7th Edition)
Ch. 3 - Prob. 3.1PCh. 3 - Prob. 3.2ACh. 3 - Prob. 3.3PCh. 3 - APPLY 3.4 The major ingredient in ordinary safety...Ch. 3 - Prob. 3.5PCh. 3 - Conceptual APPLY 3.6 Use the structural formula of...Ch. 3 - PRACTICE 3.7 How many moles arc in 5.26 g of...Ch. 3 - APPLY 3.8 When a diabetic experiences low blood...Ch. 3 - PRACTICE 3.9 Aspirin is prepared by reaction...Ch. 3 - APPLY 3.10 Refer to the balanced reaction for the...
Ch. 3 - PRACTICE 3.11 Ethyl alcohol is prepared...Ch. 3 - APPLY 3.12 (a) Diethyl ether (C4H10O), the “ether”...Ch. 3 - Conceptual PRACTICE 3.13 The following diagram...Ch. 3 - Conceptual APPLY 3.14 Draw a diagram similar to...Ch. 3 - Prob. 3.15PCh. 3 - APPLY 3.16 After lithium hydroxide is produced...Ch. 3 - PRACTICE 3.17 What is the empirical formula of the...Ch. 3 - Prob. 3.18PCh. 3 - Conceptual APPLY 3.19 Use the structural formula...Ch. 3 - PRACTICE 3.20 Menthol, a flavouring agent obtained...Ch. 3 - PRACTICE 321 Combustion analysis is performed on...Ch. 3 - APPLY 3.22 (a) Polychlorinated biphenyls (PCBs)...Ch. 3 - PRACTICE 3.23 A compound has an empirical formula...Ch. 3 - APPLY 3.24 Combustion analysis was performed on...Ch. 3 - Prob. 3.25PCh. 3 - PROBLEM 3.26 (a) Balance the reaction for...Ch. 3 - Prob. 3.27PCh. 3 - Prob. 3.28PCh. 3 - The reaction of A (red spheres) with B (blue...Ch. 3 - Prob. 3.30CPCh. 3 - Prob. 3.31CPCh. 3 - Prob. 3.32CPCh. 3 - Prob. 3.33CPCh. 3 - Prob. 3.34CPCh. 3 - Prob. 3.35CPCh. 3 - Which of the following equations are balanced? (a)...Ch. 3 - Which of the following equations are balanced?...Ch. 3 - Balance the following equations: (a)...Ch. 3 - Balance the following equations: (a) The explosion...Ch. 3 - Balance the following equations: (a)...Ch. 3 - Balance the following equations:...Ch. 3 - What are the molecular (formula) weights of the...Ch. 3 - What are the formulas of the following substances?...Ch. 3 - What are the molecular weights of the following...Ch. 3 - Prob. 3.45SPCh. 3 - How many grams are in a mole of each of the...Ch. 3 - Prob. 3.47SPCh. 3 - How many moles of ions are in 27.5 g of MgCl2?Ch. 3 - How many moles of anions are in 35.6 g of AlF3?Ch. 3 - What is the molecular weight of chloroform if...Ch. 3 - What is the molecular weight of cholesterol if...Ch. 3 - 3.52 Iron (II) sulfate, FeSO4, is prescribed for...Ch. 3 - Prob. 3.53SPCh. 3 - An average cup of coffee contains about 125 mg of...Ch. 3 - Prob. 3.55SPCh. 3 - A sample that weighs 25.12 g contains 6.0221023...Ch. 3 - Prob. 3.57SPCh. 3 - Prob. 3.58SPCh. 3 - Prob. 3.59SPCh. 3 - Prob. 3.60SPCh. 3 - An alternative method for preparing pure iron from...Ch. 3 - Prob. 3.62SPCh. 3 - Prob. 3.63SPCh. 3 - Prob. 3.64SPCh. 3 - Prob. 3.65SPCh. 3 - Prob. 3.66SPCh. 3 - Prob. 3.67SPCh. 3 - Prob. 3.68SPCh. 3 - Prob. 3.69SPCh. 3 - Prob. 3.70SPCh. 3 - 3.71 Hydrogen and chlorine react to yield hydrogen...Ch. 3 - Prob. 3.72SPCh. 3 - Prob. 3.73SPCh. 3 - Nickel (ll) sulfate, used for nickel plating, is...Ch. 3 - Prob. 3.75SPCh. 3 - Prob. 3.76SPCh. 3 - Prob. 3.77SPCh. 3 - Prob. 3.78SPCh. 3 - Prob. 3.79SPCh. 3 - Prob. 3.80SPCh. 3 - Prob. 3.81SPCh. 3 - Prob. 3.82SPCh. 3 - Prob. 3.83SPCh. 3 - Prob. 3.84SPCh. 3 - Prob. 3.85SPCh. 3 - Prob. 3.86SPCh. 3 - What are the empirical formulas of each of the...Ch. 3 - Prob. 3.88SPCh. 3 - 3.89 Coniine, a toxic substance isolated from...Ch. 3 - Prob. 3.90SPCh. 3 - Prob. 3.91SPCh. 3 - Prob. 3.92SPCh. 3 - Prob. 3.93SPCh. 3 - Prob. 3.94SPCh. 3 - Prob. 3.95SPCh. 3 - Prob. 3.96SPCh. 3 - Prob. 3.97SPCh. 3 - 3.98 The molecular weight of an organic compound...Ch. 3 - Prob. 3.99SPCh. 3 - Prob. 3.100SPCh. 3 - (a) Combustion analysis of 150.0 mg of 1, 2, 3,...Ch. 3 - Prob. 3.102CPCh. 3 - Prob. 3.103CPCh. 3 - Prob. 3.104CPCh. 3 - Prob. 3.105CPCh. 3 - Prob. 3.106CPCh. 3 - Prob. 3.107CPCh. 3 - Prob. 3.108CPCh. 3 - Ferrocene, a substance proposed for use as a...Ch. 3 - Prob. 3.110CPCh. 3 - Ethylene glycol, commonly used as automobile...Ch. 3 - Prob. 3.112CPCh. 3 - Prob. 3.113CPCh. 3 - Prob. 3.114CPCh. 3 - Prob. 3.115CPCh. 3 - A pulverized rock sample believed to be pure...Ch. 3 - Prob. 3.117CPCh. 3 - Prob. 3.118CPCh. 3 - Prob. 3.119CPCh. 3 - Prob. 3.120CPCh. 3 - Prob. 3.121CPCh. 3 - Prob. 3.122CPCh. 3 - Prob. 3.123CPCh. 3 - Prob. 3.124CPCh. 3 - Prob. 3.125CPCh. 3 - Prob. 3.126CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forwardQuestion: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forwardCalculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3Cu+ (aq) + Cro²¯ (aq) +4H₂O (1) → 3Cu²+ (aq) +Cr(OH)3 (s)+5OH˜¯ (aq) 0 kJ ☐ x10 00. 18 Ararrow_forward
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 241.7 mL of a 0.4900M solution of methylamine (CH3NH2) with a 0.7800M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 17.7 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☑ ? 18 Ararrow_forwardThe following is two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 223.4 148.7 353.7 278.2 334.6 268.7 305.6 234.4 340.0 262.7 304.3 283.2 244.7 143.6 QUESTION: For both groups of data calculate the answers attached in the image.arrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0mmol/L 262.7mmol/L QUESTION: For both groups (Regular & Salt Reduced tomato sauce) of data provide answers to the following calculations below: 1. Standard Deviation (Sx) 2. T Values (t0.05,4) 3. 95% Confidence Interval (mmol/L) 4. [Na+] (mg/100 mL) 5. 95% Confidence Interval (mg/100 mL)arrow_forward
- If we have leucine (2-amino-4-methylpentanoic acid), alanine (2-aminopropanoic acid) and phenylalanine (2-amino-3-phenylpropanoic acid), indicate the tripeptides that can be formed (use the abbreviated symbols Leu., Ala and Phe).arrow_forwardBriefly state why trifluoroacetic acid is more acidic than acetic acid.arrow_forwardExplain why acid chlorides are more reactive than amides in reactions with nucleophiles.arrow_forward
- Calculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 101.7 mL of a 0.3500M solution of piperidine (C5H10NH) with a 0.05700M solution of HClO4. The pK of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 682.9 mL of the HClO solution to it. 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO solution added. 4 Round your answer to 2 decimal places. pH = .11 00. 18 Ararrow_forwardThe following is a two groups (Regular tomato sauce & Salt Reduced Tomato Sauce) of data recorded by a team analysising salt content in tomato sauce using the MOHR titration method: Regular Tomato Sauce Salt Reduced Tomato Sauce 340.0 262.7 QUESTION: For both groups of data provide answers to the calculations attached in the imagearrow_forward7. Concentration and uncertainty in the estimate of concentration (class data) Class mean for sample (Regular) |[Cl-] (mmol/L) class mean Sn za/2 95% Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Confidence Interval (mg/100 mL)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Step by Step Stoichiometry Practice Problems | How to Pass ChemistryMole Conversions Made Easy: How to Convert Between Grams and Moles; Author: Ketzbook;https://www.youtube.com/watch?v=b2raanVWU6c;License: Standard YouTube License, CC-BY