
(a)
Interpretation:
The symbol and name for the element contains only two
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their
Answer to Problem 3.28E
The name of the element that contains only two
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains only two
The name of the element that contains only two
(b)
Interpretation:
The symbol and name for the element contains an unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains an unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains an unpaired
The name of the element that contains an unpaired
(c)
Interpretation:
The symbol and name for the element contains two unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the elements that contain two unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains two unpaired
The element having electronic configuration
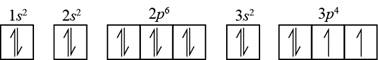
Figure 1
The element having electronic configuration
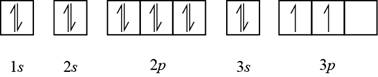
Figure 2
Hence, sulfur and silicon elements contain two unpaired
The name of the elements that contain two unpaired
(d)
Interpretation:
The symbol and name for the element contains three
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains three
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that three
The name of the element that contains three
(e)
Interpretation:
The symbol and name for the element contains three unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains three unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains three unpaired
The element having electronic configuration
![]()
Figure 3
The element having electronic configuration
![]()
Figure 4
Hence, vanadium and cobalt elements contain three unpaired
The name of the element that contains three unpaired
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- 1. For each of the reaction "railroads" below, you are either asked to give the structure(s) of the starting material(s) or product(s), or provide reagents/conditions to accomplish the transformation, as indicated by the boxes. a. NaOMe H+ .CO,H HO₂C MeOH (excess) MeOH H3C Br يع CH3 1. LiAlH4 2. H3O+ 3. PBг3 H3C 1. Et-Li 2. H3O+ -CO₂Me -CO₂Me OH CH3 CH3 ল CH3arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe ག1, ད།་, - + H You can draw 1 and 2 in any arrangement you like. 2 work up Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Parrow_forwardWhat is the total energy cost associated with the compound below adopting the shown conformation? CH3 HH DH CH3arrow_forward
- ΗΝ, Draw Final Product C cyclohexanone pH 4-5 Edit Enamine H3O+ CH3CH2Br THF, reflux H Edit Iminium Ionarrow_forwardHow many hydrogen atoms are connected to the indicated carbon atom?arrow_forwardIdentify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forward
- Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forwardRank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forward
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





