
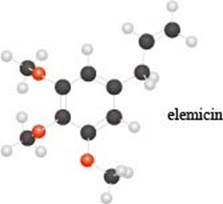
a. Identify the functional groups in the ball-and-stick model of elemicin, a compound partly responsible f or the flavor and fragrance of nutmeg.
b. Draw a skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point.
c. Label all electrophilic carbon atoms.
(a)
Interpretation: The functional groups in the ball-and-stick model of elemicin are to be identified.
Concept introduction: An atom or a group of atoms which are responsible for characteristic physical and chemical properties of the compound are collectively known as functional groups. The functional groups are the most reactive part present in the molecule.
Answer to Problem 31P
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
Explanation of Solution
The given molecule is,
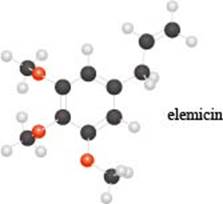
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of elemicin is,
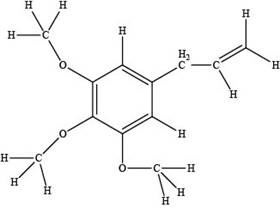
Figure 2
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
(b)
Interpretation: The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is to be drawn.
Concept introduction: The isomers which have same molecular formula but different connectivity of atoms are constitutional isomers.
The temperature at which the vapour pressure of a substance becomes equal to the pressure surrounding the liquid is boiling point. The boiling point depends on the intermolecular forces. Stronger the intermolecular force, higher is the boiling point.
The temperature at which the solid converts to its liquid phase is melting point. Stronger the intermolecular force, higher is the boiling point.
Answer to Problem 31P
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown below.
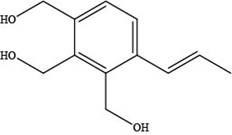
Explanation of Solution
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is,

Figure 3
This structure contains alcohol groups. This structure exhibits intermolecular hydrogen bonding due to the presence of hydrogen atoms bonded to oxygen atom. Hydrogen bonding is strongest intermolecular forces.
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown in Figure 3.
(c)
Interpretation: All electrophilic carbon atoms are to be labeled.
Concept introduction: An electron deficient due to hetero atoms or pi bonds or both is electrophilic site and an electron rich site due to hetero atoms or pi bonds or both is nucleophilic site.
Answer to Problem 31P
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Explanation of Solution
An oxygen atom is more electronegative than carbon atom which makes the carbon atom attached to oxygen atom electron deficient and electrophilic site as shown below.
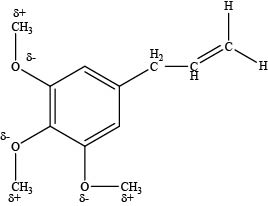
Figure 4
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Want to see more full solutions like this?
Chapter 3 Solutions
ORGANIC CHEMISTRY - LOOSELEAF W/CONNECT
- Provide steps and explanation please.arrow_forwardDraw a structural formula for the major product of the acid-base reaction shown. H 0 N + HCI (1 mole) CH3 N' (1 mole) CH3 You do not have to consider stereochemistry. ● • Do not include counter-ions, e.g., Na+, I, in your answer. . In those cases in which there are two reactants, draw only the product from 989 CH3 344 ? [Farrow_forwardQuestion 15 What is the major neutral organic product for the following sequence? 1. POCI₂ pyridine ? 2. OsO4 OH 3. NaHSO Major Organic Product ✓ OH OH 'OH OH 'OH 'CIarrow_forward
- Could you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but color-coded or step by step so I can understand it better? Thank you!arrow_forwardCould you please solve the first problem in this way and present it similarly but (color-coded) and step by step so I can understand it better? Thank you! I want to see what they are doingarrow_forward
- Can you please help mne with this problem. Im a visual person, so can you redraw it, potentislly color code and then as well explain it. I know im given CO2 use that to explain to me, as well as maybe give me a second example just to clarify even more with drawings (visuals) and explanations.arrow_forwardPart 1. Aqueous 0.010M AgNO 3 is slowly added to a 50-ml solution containing both carbonate [co32-] = 0.105 M and sulfate [soy] = 0.164 M anions. Given the ksp of Ag2CO3 and Ag₂ soy below. Answer the ff: Ag₂ CO3 = 2 Ag+ caq) + co} (aq) ksp = 8.10 × 10-12 Ag₂SO4 = 2Ag+(aq) + soy² (aq) ksp = 1.20 × 10-5 a) which salt will precipitate first? (b) What % of the first anion precipitated will remain in the solution. by the time the second anion starts to precipitate? (c) What is the effect of low pH (more acidic) condition on the separate of the carbonate and sulfate anions via silver precipitation? What is the effect of high pH (more basic)? Provide appropriate explanation per answerarrow_forwardPart 4. Butanoic acid (ka= 1.52× 10-5) has a partition coefficient of 3.0 (favors benzene) when distributed bet. water and benzene. What is the formal concentration of butanoic acid in each phase when 0.10M aqueous butanoic acid is extracted w❘ 25 mL of benzene 100 mL of a) at pit 5.00 b) at pH 9.00arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

