
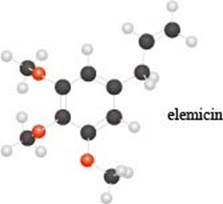
a. Identify the functional groups in the ball-and-stick model of elemicin, a compound partly responsible f or the flavor and fragrance of nutmeg.
b. Draw a skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point.
c. Label all electrophilic carbon atoms.
(a)
Interpretation: The functional groups in the ball-and-stick model of elemicin are to be identified.
Concept introduction: An atom or a group of atoms which are responsible for characteristic physical and chemical properties of the compound are collectively known as functional groups. The functional groups are the most reactive part present in the molecule.
Answer to Problem 31P
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
Explanation of Solution
The given molecule is,
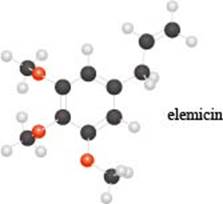
Figure 1
The red coloured balls have two bonds. So, these are the oxygen atoms. Black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of elemicin is,
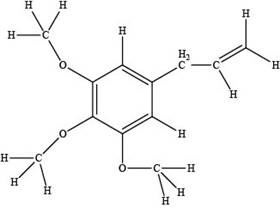
Figure 2
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
The functional groups present in the ball-and-stick model of elemicin are ether and alkene group.
(b)
Interpretation: The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is to be drawn.
Concept introduction: The isomers which have same molecular formula but different connectivity of atoms are constitutional isomers.
The temperature at which the vapour pressure of a substance becomes equal to the pressure surrounding the liquid is boiling point. The boiling point depends on the intermolecular forces. Stronger the intermolecular force, higher is the boiling point.
The temperature at which the solid converts to its liquid phase is melting point. Stronger the intermolecular force, higher is the boiling point.
Answer to Problem 31P
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown below.
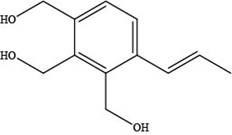
Explanation of Solution
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is,

Figure 3
This structure contains alcohol groups. This structure exhibits intermolecular hydrogen bonding due to the presence of hydrogen atoms bonded to oxygen atom. Hydrogen bonding is strongest intermolecular forces.
The skeletal structure of a constitutional isomer of elemicin that should have a higher boiling point and melting point is shown in Figure 3.
(c)
Interpretation: All electrophilic carbon atoms are to be labeled.
Concept introduction: An electron deficient due to hetero atoms or pi bonds or both is electrophilic site and an electron rich site due to hetero atoms or pi bonds or both is nucleophilic site.
Answer to Problem 31P
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Explanation of Solution
An oxygen atom is more electronegative than carbon atom which makes the carbon atom attached to oxygen atom electron deficient and electrophilic site as shown below.
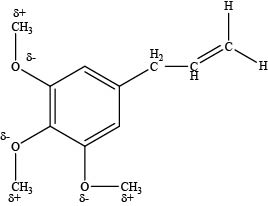
Figure 4
The carbon atoms attached to the oxygen atom in ether group are electrophilic in nature.
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry (6th Edition)
- Need help with 14 and 15. 14. bromobenzene + (CHs),CuLi + THF / -78° followed by water quench is a. toluene else!! b. xylene c. cumene d. styrene e. something 15. When cumene + H,SO, / Na,Cr, 0,/water are mixed (refluxed) what is produced? a. 2-phenylpropanol phenol e. styrene b. benzoic acid c. no reaction!arrow_forwardWhich of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.) Group of answer choices px dxz dx2-y2 py dxy sarrow_forwardWhich of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices Li-Na-K Al-Ga-In Cr-Mo-W K-Rb-Csarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forward
- Give the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forwardCalculate the residence time of strontium (Sr2+) in the world ocean, given that the average concentration of strontium in the world’s rivers is approximately 0.87 µmol L-1 (5 pts).arrow_forwardA package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forward
- How is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forwardPart II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

