
Concept explainers
The dimethyldithiocarbamate ion,
![Chapter 3, Problem 3.1P, The dimethyldithiocarbamate ion, [S2CN( CH 3 )2]- has the following skeletal structure a. Give the](http://dev-ingestion-image-output.s3-website-us-east-1.amazonaws.com/9780321811059/Chapter-3/images/html_11059-3-3.1p_image001.jpg)
a. Give the important resonance structures of this ion, including any formal charges wherenecessary. Select the resonance structure likely to provide the best description of this ion.
b. Repeat for the dimethylthiocarbamate ion,
(a)
Interpretation: The most important resonance structures of
Concept introduction: Formula to calculate the formal charge of the atom is as follows:
More important resonance structure selected as follows:
1. Always prefer smaller formal charges over the larger formal charge.
2. Always prefer the different formal charges on adjacent atoms instead of the same nonzero formal charge.
3. A more negative formal charge must be located on the most electronegative atom.
Explanation of Solution
Nitrogen has five valence electrons,carbon has four valence electrons and sulfur has six valence electrons. The resonance structure of
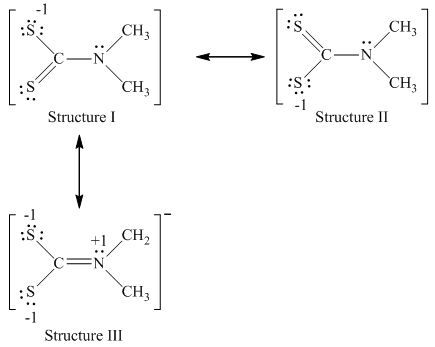
Since structure I and II have complete octet with least negative charge that resonates on sulfur, hence, most important structures are structure I and II.
(b)
Interpretation: The most important resonance structures of
Concept introduction: Formula to calculate the formal charge of the atom is as follows:
More important resonance structure selected as follows:
1. Always prefer smaller formal charges over the larger formal charge.
2. Always prefer the different formal charges on adjacent atoms instead of the same non-zero formal charge.
3. A more negative formal charge must be located on the most electronegative atom.
Explanation of Solution
Nitrogen has five valence electrons,carbon has four valence electrons and sulfur has six valence electrons. The resonance structure of
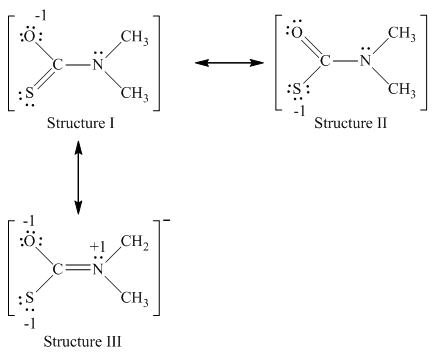
Since structure I has complete octet with least negative charge which exists on more electronegative atom that is oxygen,hence, most important structures is structure I.
Want to see more full solutions like this?
Chapter 3 Solutions
Inorganic Chemistry
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry - 4th edition
Physics of Everyday Phenomena
Campbell Biology (11th Edition)
Cosmic Perspective Fundamentals
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



