
Concept explainers
Which
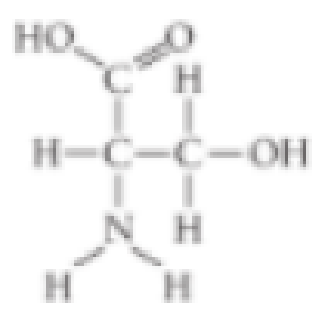
- A. carboxyl
- B. sulfhydryl
- C. hydroxyl
- D. amino
Introduction:
The different properties of an organic molecule not only depend upon the carbon skeleton’s arrangement, it also depends on the different chemical groups attached to the skeleton.
Answer to Problem 1TYU
Correct answer:
The functional group that is not present in the given molecule is sulfhydryl group.
Therefore, option (B) is correct.
Explanation of Solution
Reason for the correct statement:
Sulfhydryl group is the functional group that comprises of a molecule of sulfur. Hence, the given structure does not have a sulfhydryl group.
Option (B) is given as “sulfhydryl”.
The “functional group that is not present in the given molecule is sulfhydryl group”, it is the right answer.
Hence, the option (B) is correct.
Reasons for the incorrect statements:
Option (A) is given as “carboxyl”.
The carboxyl functional group is present in the given molecule. So, it is a wrong answer.
Option (C) is given as “hydroxyl”.
The hydroxyl functional group is present in the given molecule. So, it is a wrong answer.
Option (D) is given as “amino”.
The amino group is present in the given molecule. So, it is a wrong answer.
Hence, options (A), (C), and (D) are incorrect.
The functional groups such as carboxyl, hydroxyl, and amino are present in the molecule, whereas the sulfhydryl group is absent in the given molecule.
Want to see more full solutions like this?
Chapter 3 Solutions
ETEXT CAMPBELL BIOLOGY IN FOCUS INSTANT
Additional Science Textbook Solutions
Biological Science (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
Chemistry
Laboratory Manual For Human Anatomy & Physiology
SEELEY'S ANATOMY+PHYSIOLOGY
Biology: Life on Earth with Physiology (11th Edition)
- Ch.23 How is Salmonella able to cross from the intestines into the blood? A. it is so small that it can squeeze between intestinal cells B. it secretes a toxin that induces its uptake into intestinal epithelial cells C. it secretes enzymes that create perforations in the intestine D. it can get into the blood only if the bacteria are deposited directly there, that is, through a puncture — Which virus is associated with liver cancer? A. hepatitis A B. hepatitis B C. hepatitis C D. both hepatitis B and C — explain your answer thoroughlyarrow_forwardCh.21 What causes patients infected with the yellow fever virus to turn yellow (jaundice)? A. low blood pressure and anemia B. excess leukocytes C. alteration of skin pigments D. liver damage in final stage of disease — What is the advantage for malarial parasites to grow and replicate in red blood cells? A. able to spread quickly B. able to avoid immune detection C. low oxygen environment for growth D. cooler area of the body for growth — Which microbe does not live part of its lifecycle outside humans? A. Toxoplasma gondii B. Cytomegalovirus C. Francisella tularensis D. Plasmodium falciparum — explain your answer thoroughlyarrow_forwardCh.22 Streptococcus pneumoniae has a capsule to protect it from killing by alveolar macrophages, which kill bacteria by… A. cytokines B. antibodies C. complement D. phagocytosis — What fact about the influenza virus allows the dramatic antigenic shift that generates novel strains? A. very large size B. enveloped C. segmented genome D. over 100 genes — explain your answer thoroughlyarrow_forward
- What is this?arrow_forwardMolecular Biology A-C components of the question are corresponding to attached image labeled 1. D component of the question is corresponding to attached image labeled 2. For a eukaryotic mRNA, the sequences is as follows where AUGrepresents the start codon, the yellow is the Kozak sequence and (XXX) just represents any codonfor an amino acid (no stop codons here). G-cap and polyA tail are not shown A. How long is the peptide produced?B. What is the function (a sentence) of the UAA highlighted in blue?C. If the sequence highlighted in blue were changed from UAA to UAG, how would that affecttranslation? D. (1) The sequence highlighted in yellow above is moved to a new position indicated below. Howwould that affect translation? (2) How long would be the protein produced from this new mRNA? Thank youarrow_forwardMolecular Biology Question Explain why the cell doesn’t need 61 tRNAs (one for each codon). Please help. Thank youarrow_forward
- Molecular Biology You discover a disease causing mutation (indicated by the arrow) that alters splicing of its mRNA. This mutation (a base substitution in the splicing sequence) eliminates a 3’ splice site resulting in the inclusion of the second intron (I2) in the final mRNA. We are going to pretend that this intron is short having only 15 nucleotides (most introns are much longer so this is just to make things simple) with the following sequence shown below in bold. The ( ) indicate the reading frames in the exons; the included intron 2 sequences are in bold. A. Would you expected this change to be harmful? ExplainB. If you were to do gene therapy to fix this problem, briefly explain what type of gene therapy youwould use to correct this. Please help. Thank youarrow_forwardMolecular Biology Question Please help. Thank you Explain what is meant by the term “defective virus.” Explain how a defective virus is able to replicate.arrow_forwardMolecular Biology Explain why changing the codon GGG to GGA should not be harmful. Please help . Thank youarrow_forward
- Stage Percent Time in Hours Interphase .60 14.4 Prophase .20 4.8 Metaphase .10 2.4 Anaphase .06 1.44 Telophase .03 .72 Cytukinesis .01 .24 Can you summarize the results in the chart and explain which phases are faster and why the slower ones are slow?arrow_forwardCan you circle a cell in the different stages of mitosis? 1.prophase 2.metaphase 3.anaphase 4.telophase 5.cytokinesisarrow_forwardWhich microbe does not live part of its lifecycle outside humans? A. Toxoplasma gondii B. Cytomegalovirus C. Francisella tularensis D. Plasmodium falciparum explain your answer thoroughly.arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





