
Concept explainers
Which
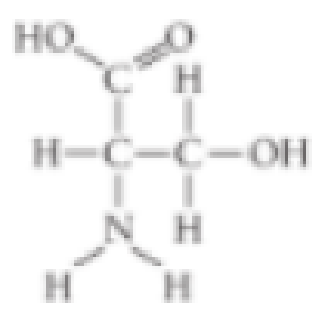
- A. carboxyl
- B. sulfhydryl
- C. hydroxyl
- D. amino
Introduction:
The different properties of an organic molecule not only depend upon the carbon skeleton’s arrangement, it also depends on the different chemical groups attached to the skeleton.
Answer to Problem 1TYU
Correct answer:
The functional group that is not present in the given molecule is sulfhydryl group.
Therefore, option (B) is correct.
Explanation of Solution
Reason for the correct statement:
Sulfhydryl group is the functional group that comprises of a molecule of sulfur. Hence, the given structure does not have a sulfhydryl group.
Option (B) is given as “sulfhydryl”.
The “functional group that is not present in the given molecule is sulfhydryl group”, it is the right answer.
Hence, the option (B) is correct.
Reasons for the incorrect statements:
Option (A) is given as “carboxyl”.
The carboxyl functional group is present in the given molecule. So, it is a wrong answer.
Option (C) is given as “hydroxyl”.
The hydroxyl functional group is present in the given molecule. So, it is a wrong answer.
Option (D) is given as “amino”.
The amino group is present in the given molecule. So, it is a wrong answer.
Hence, options (A), (C), and (D) are incorrect.
The functional groups such as carboxyl, hydroxyl, and amino are present in the molecule, whereas the sulfhydryl group is absent in the given molecule.
Want to see more full solutions like this?
Chapter 3 Solutions
CAMPBELL BIOLOGY IN FOCUS-W/MASTR.BIO.
Additional Science Textbook Solutions
Biological Science (6th Edition)
College Physics: A Strategic Approach (3rd Edition)
Chemistry
Laboratory Manual For Human Anatomy & Physiology
SEELEY'S ANATOMY+PHYSIOLOGY
Biology: Life on Earth with Physiology (11th Edition)
- Alleles at the P locus control seed color. Plants which are pp have white seeds, white flowers and no pigment in vegetative parts. Plants which are P_ have black seeds, purple flowers and may have varying degrees of pigment on stems and leaves. Seed color can be assessed, visually, based on if the seed is white or not white A gene for mold resistance has been reported and we want to determine its inheritance and whether it is linked to P. For the purposes of this exercise, we will assume that resistance is controlled by a single locus M, and M_ plants are resistant and mm plants are susceptible. Resistance can be measured, under greenhouse conditions, 2 weeks after planting, by injecting each seedling with a spore suspension. After two weeks, the seedlings can be rated as resistant or susceptible, based on whether or not tissue is actively sporulating. For this exercise we will use seed and data from the F10 generation of a recombinant inbred population produced using single seed…arrow_forwardcan you help? I think its B but not surearrow_forwardSkip to main content close Homework Help is Here – Start Your Trial Now! arrow_forward search SEARCH ASK Human Anatomy & Physiology (11th Edition)BUY Human Anatomy & Physiology (11th Edition) 11th Edition ISBN: 9780134580999 Author: Elaine N. Marieb, Katja N. Hoehn Publisher: PEARSON 1 The Human Body: An Orientation expand_moreChapter 1 : The Human Body: An Orientation Chapter Questions expand_moreSection: Chapter Questions Problem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,... format_list_bulletedProblem 1RQ: The correct sequence of levels forming the structural hierarchy is A. (a) organ, organ system,... See similar textbooks Bartleby Related Questions Icon Related questions Bartleby Expand Icon bartleby Concept explainers bartleby Question Draw a replication bubble with two replication forks.blue lines are DNA single strands and red lines are RNA single strands.indicate all 3' and 5’ ends on all DNA single…arrow_forward
- Provide an answerarrow_forwardQuestion 4 1 pts Which of the following would be most helpful for demonstrating alternative splicing for a new organism? ○ its proteome and its transcriptome only its transcriptome only its genome its proteome and its genomearrow_forwardIf the metabolic scenario stated with 100 mM of a sucrose solution, how much ATP would be made then during fermentation?arrow_forward
- What is agricuarrow_forwardWhen using the concept of "a calorie in is equal to a calorie out" how important is the quality of the calories?arrow_forwardWhat did the Cre-lox system used in the Kikuchi et al. 2010 heart regeneration experiment allow researchers to investigate? What was the purpose of the cmlc2 promoter? What is CreER and why was it used in this experiment? If constitutively active Cre was driven by the cmlc2 promoter, rather than an inducible CreER system, what color would you expect new cardiomyocytes in the regenerated area to be no matter what? Why?arrow_forward
- What kind of organ size regulation is occurring when you graft multiple organs into a mouse and the graft weight stays the same?arrow_forwardWhat is the concept "calories consumed must equal calories burned" in regrads to nutrition?arrow_forwardYou intend to insert patched dominant negative DNA into the left half of the neural tube of a chick. 1) Which side of the neural tube would you put the positive electrode to ensure that the DNA ends up on the left side? 2) What would be the internal (within the embryo) control for this experiment? 3) How can you be sure that the electroporation method itself is not impacting the embryo? 4) What would you do to ensure that the electroporation is working? How can you tell?arrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
 Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College





