
FOUND.OF COLLEGE CHEMISTRY
15th Edition
ISBN: 9781119234555
Author: Hein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 1PE
Interpretation Introduction
Interpretation:
The diatomic molecules have to be identified.
Concept Introduction:
Diatomic molecules: Those molecules that contain exactly two similar atoms (or) two different atoms are called as diatomic molecules. The names and formulas of diatomic molecules are given as,
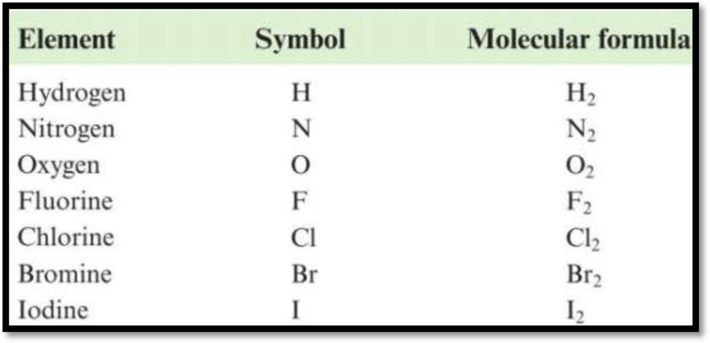
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please sirrr soollveee these parts pleaseeee and thank youuuuu
Please sirrr soollveee these parts pleaseeee and thank youuuuu, don't solve it by AI plleeaasseee
Please sirrr soollveee these parts pleaseeee and thank youuuuu
Chapter 3 Solutions
FOUND.OF COLLEGE CHEMISTRY
Ch. 3.1 - Prob. 3.1PCh. 3.2 - Prob. 3.2PCh. 3.2 - Prob. 3.3PCh. 3.2 - Prob. 3.4PCh. 3.3 - Prob. 3.5PCh. 3.3 - Prob. 3.6PCh. 3 - Prob. 1RQCh. 3 - Prob. 2RQCh. 3 - Prob. 3RQCh. 3 - Prob. 4RQ
Ch. 3 - Prob. 5RQCh. 3 - Prob. 6RQCh. 3 - Prob. 7RQCh. 3 - Prob. 8RQCh. 3 - Prob. 9RQCh. 3 - Prob. 10RQCh. 3 - Prob. 11RQCh. 3 - Prob. 12RQCh. 3 - Prob. 13RQCh. 3 - Prob. 14RQCh. 3 - Prob. 15RQCh. 3 - Prob. 16RQCh. 3 - Prob. 17RQCh. 3 - Prob. 1PECh. 3 - Prob. 2PECh. 3 - Prob. 3PECh. 3 - Prob. 4PECh. 3 - Prob. 5PECh. 3 - Prob. 6PECh. 3 - Prob. 7PECh. 3 - Prob. 8PECh. 3 - Prob. 9PECh. 3 - Prob. 10PECh. 3 - Prob. 11PECh. 3 - Prob. 12PECh. 3 - Prob. 13PECh. 3 - Prob. 14PECh. 3 - Prob. 15PECh. 3 - Prob. 16PECh. 3 - Prob. 17PECh. 3 - Prob. 18PECh. 3 - Prob. 19PECh. 3 - Prob. 20PECh. 3 - Prob. 21PECh. 3 - Prob. 22PECh. 3 - Prob. 23PECh. 3 - Prob. 24PECh. 3 - Prob. 25PECh. 3 - Prob. 26PECh. 3 - Prob. 27AECh. 3 - Prob. 28AECh. 3 - Prob. 29AECh. 3 - Prob. 30AECh. 3 - Prob. 31AECh. 3 - Prob. 32AECh. 3 - Prob. 33AECh. 3 - Prob. 34AECh. 3 - Prob. 35AECh. 3 - Prob. 36AECh. 3 - Prob. 38AECh. 3 - Prob. 39AECh. 3 - Prob. 40AECh. 3 - Prob. 41AECh. 3 - Prob. 42AECh. 3 - Prob. 43AECh. 3 - Prob. 44AECh. 3 - Prob. 45CECh. 3 - Prob. 46CE
Knowledge Booster
Similar questions
- 4. Read paragraph 4.15 from your textbook, use your calculated lattice energy values for CuO, CuCO3 and Cu(OH)2 an explain thermal decomposition reaction of malachite: Cu2CO3(OH)2 →2CuO + H2O + CO2 (3 points)arrow_forwardPlease sirrr soollveee these parts pleaseeee and thank youuuuuarrow_forwardIII O Organic Chemistry Using wedges and dashes in skeletal structures Draw a skeletal ("line") structure for each of the molecules below. Be sure your structures show the important difference between the molecules. key O O O O O CHON Cl jiii iiiiiiii You can drag the slider to rotate the molecules. Explanation Check Click and drag to start drawing a structure. Q Search X G ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use F 3 W C 3/5arrow_forward
- 3. Use Kapustinskii's equation and data from Table 4.10 in your textbook to calculate lattice energies of Cu(OH)2 and CuCO3 (4 points)arrow_forward2. Copper (II) oxide crystalizes in monoclinic unit cell (included below; blue spheres 2+ represent Cu²+, red - O²-). Use Kapustinski's equation (4.5) to calculate lattice energy for CuO. You will need some data from Resource section of your textbook (p.901). (4 points) CuOarrow_forwardWhat is the IUPAC name of the following compound? OH (2S, 4R)-4-chloropentan-2-ol O (2R, 4R)-4-chloropentan-2-ol O (2R, 4S)-4-chloropentan-2-ol O(2S, 4S)-4-chloropentan-2-olarrow_forward
- Use the reaction coordinate diagram to answer the below questions. Type your answers into the answer box for each question. (Watch your spelling) Energy A B C D Reaction coordinate E A) Is the reaction step going from D to F endothermic or exothermic? A F G B) Does point D represent a reactant, product, intermediate or transition state? A/ C) Which step (step 1 or step 2) is the rate determining step? Aarrow_forward1. Using radii from Resource section 1 (p.901) and Born-Lande equation, calculate the lattice energy for PbS, which crystallizes in the NaCl structure. Then, use the Born-Haber cycle to obtain the value of lattice energy for PbS. You will need the following data following data: AH Pb(g) = 196 kJ/mol; AHƒ PbS = −98 kJ/mol; electron affinities for S(g)→S¯(g) is -201 kJ/mol; S¯(g) (g) is 640kJ/mol. Ionization energies for Pb are listed in Resource section 2, p.903. Remember that enthalpies of formation are calculated beginning with the elements in their standard states (S8 for sulfur). The formation of S2, AHF: S2 (g) = 535 kJ/mol. Compare the two values, and explain the difference. (8 points)arrow_forwardIn the answer box, type the number of maximum stereoisomers possible for the following compound. A H H COH OH = H C Br H.C OH CHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning