
FOUND.OF COLLEGE CHEMISTRY
15th Edition
ISBN: 9781119234555
Author: Hein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 3, Problem 2PE
Interpretation Introduction
Interpretation:
The diatomic molecules have to be identified.
Concept Introduction:
Diatomic molecules: Those molecules that contain exactly two similar atoms (or) two different atoms are called as diatomic molecules. The names and formulas of diatomic molecules are given as,
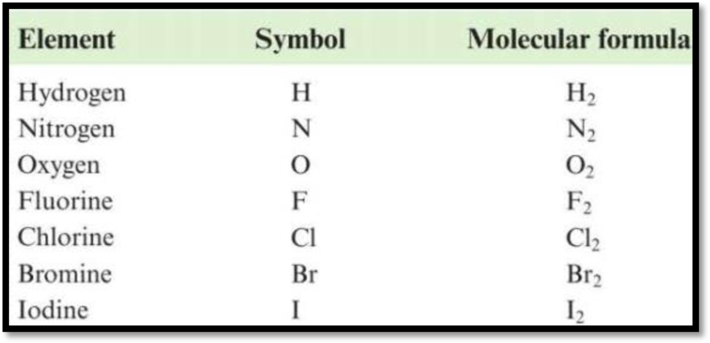
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you explain step by step behind what the synthetic strategy would be?
Please explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!
2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is
present.
.OH
HO
H₂N
OH
Chapter 3 Solutions
FOUND.OF COLLEGE CHEMISTRY
Ch. 3.1 - Prob. 3.1PCh. 3.2 - Prob. 3.2PCh. 3.2 - Prob. 3.3PCh. 3.2 - Prob. 3.4PCh. 3.3 - Prob. 3.5PCh. 3.3 - Prob. 3.6PCh. 3 - Prob. 1RQCh. 3 - Prob. 2RQCh. 3 - Prob. 3RQCh. 3 - Prob. 4RQ
Ch. 3 - Prob. 5RQCh. 3 - Prob. 6RQCh. 3 - Prob. 7RQCh. 3 - Prob. 8RQCh. 3 - Prob. 9RQCh. 3 - Prob. 10RQCh. 3 - Prob. 11RQCh. 3 - Prob. 12RQCh. 3 - Prob. 13RQCh. 3 - Prob. 14RQCh. 3 - Prob. 15RQCh. 3 - Prob. 16RQCh. 3 - Prob. 17RQCh. 3 - Prob. 1PECh. 3 - Prob. 2PECh. 3 - Prob. 3PECh. 3 - Prob. 4PECh. 3 - Prob. 5PECh. 3 - Prob. 6PECh. 3 - Prob. 7PECh. 3 - Prob. 8PECh. 3 - Prob. 9PECh. 3 - Prob. 10PECh. 3 - Prob. 11PECh. 3 - Prob. 12PECh. 3 - Prob. 13PECh. 3 - Prob. 14PECh. 3 - Prob. 15PECh. 3 - Prob. 16PECh. 3 - Prob. 17PECh. 3 - Prob. 18PECh. 3 - Prob. 19PECh. 3 - Prob. 20PECh. 3 - Prob. 21PECh. 3 - Prob. 22PECh. 3 - Prob. 23PECh. 3 - Prob. 24PECh. 3 - Prob. 25PECh. 3 - Prob. 26PECh. 3 - Prob. 27AECh. 3 - Prob. 28AECh. 3 - Prob. 29AECh. 3 - Prob. 30AECh. 3 - Prob. 31AECh. 3 - Prob. 32AECh. 3 - Prob. 33AECh. 3 - Prob. 34AECh. 3 - Prob. 35AECh. 3 - Prob. 36AECh. 3 - Prob. 38AECh. 3 - Prob. 39AECh. 3 - Prob. 40AECh. 3 - Prob. 41AECh. 3 - Prob. 42AECh. 3 - Prob. 43AECh. 3 - Prob. 44AECh. 3 - Prob. 45CECh. 3 - Prob. 46CE
Knowledge Booster
Similar questions
- consider the rate of the reaction below to be r. Whats the rate after each reaction? Br + NaCN CN + NaBr a. Double the concentration of alkyl bromide b. Halve the concentration of the electrophile & triple concentration of cyanide c. Halve the concentration of alkyl chloridearrow_forwardPredict the organic reactant that is involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forward
- What is the organic molecule X of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardWhat are is the organic molecule X and product Y of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Without using graphs, calculate the order of the reaction. t/s [R]/(mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forward
- Predict the organic products that form in the reaction below, and draw the skeletal ("line") structures of the missing organic products. Please include all steps & drawings & explanations.arrow_forwardWhat are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardWhat are the products of the following acetal hydrolysis? Please draw a skeletal line structure and include a detailed explanation and drawing of how the mechanism proceeds. Please include any relevant information that is needed to understand the process of acetal hydrolysis.arrow_forward
- What would happen if you added the HCI to the Grignard reagent before adding benzophenone? Draw a reaction mechanism to support your answer.arrow_forwardAt 300 K, in the decomposition reaction of a reactant R into products, several measurements of the concentration of R over time have been made (see table). Calculate the order of the reaction. t/s [R]/ (mol L-1) 0 0,5 171 0,16 720 0,05 1400 0,027arrow_forwardWrite the correct IUPAC names of the molecules in the picturearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning