
Concept explainers
a)
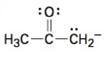
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is two.
is two.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The anion has a carbon atom doubly bonded to an oxygen and singly bonded to an adjacent carbon atom bearing negative charge. Using the nonbonding electrons on the negatively charged carbon atom and the π bond one more structure can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has two resonance forms.
The maximum resonance structures possible for the species  are two.
are two.
b)
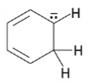
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The anion given has a carbon atom doubly bonded to another carbon and singly bonded to an adjacent carbon atom bearing negative charge. Another double bond also is present. Using the nonbonding electrons on the negatively charged carbon atom and the π bonds two more structures can be drawn as shown, without change in position or hybridization of any atom. Hence the species given has three resonance forms.
Conclusion:
The maximum resonance structures possible for the species  are three.
are three.
The maximum resonance structures possible for the species  are three.
are three.
c)
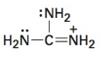
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The cation given has a carbon doubly bonded to positively charged nitrogen and singly bonded to two more nitrogens each with a pair of nonbonding electrons. Using the nonbonding electrons on the nitrogens and the π bond in C=N, two more structures, as shown, can be drawn without change in position or hybridization of any atom. Hence the species given has three resonance forms.
The maximum resonance structures possible for the species  are three.
are three.
d)
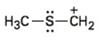
Interpretation:
To draw the maximum resonance structures possible for the species
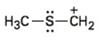
Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species
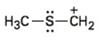
Answer to Problem 38AP
The maximum resonance structures possible for the species 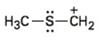 is two
is two
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The cation given has a carbon atom singly bonded to a sulfur atom which has two lone pairs of electrons. Using the nonbonding electrons on the sulfur atom one more structure can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has two resonance forms.
The maximum resonance structures possible for the species 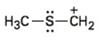 are two.
are two.
e)
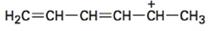
Interpretation:
To draw the maximum resonance structures possible for the species
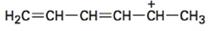
Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species
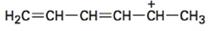
Answer to Problem 38AP
The maximum resonance structures possible for the species 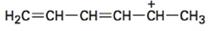 is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. In the cation given, the positively charged carbon is attached a carbon chain that contains two conjugated double bonds. Using the two π bonds two more structures can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has three resonance forms.
The maximum resonance structures possible for the species 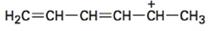 are three.
are three.
Want to see more full solutions like this?
Chapter 2 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
- О δα HO- H -Br δα HO-- + + -Br [B] 8+ HO- -Br δα नarrow_forward1/2 - 51% + » GAY Organic Reactions Assignment /26 Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted. H3C 1. 2. CH3 A Acid OH Type of Reaction: NH Type of Reaction: + H₂O Catalyst + HBr 3. Type of Reaction: H3C 4. Type Reaction: 5. H3C CH2 + H2O OH + [0] CH3 Type of Reaction: 6. OH CH3 HO CH3 + Type of Reaction: 7. Type of Reaction: + [H]arrow_forwardhumbnai Concentration Terms[1].pdf ox + New Home Edit Sign in Comment Convert Page Fill & Sign Protect Tools Batch +WPS A Free Trial Share Inter Concreting Concentration forms. Hydrogen peroxide is a powerful oxidizing agent wed in concentrated solution in rocket fuels and in dilute solution as a hair bleach. An aqueous sulation of H2O2 is 30% by mass and has density of #liligime calculat the Ⓒmolality ⑥mole fraction of molarity. 20 9. B. A sample of Commercial Concentrated hydrochloric ETarrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forward
- Draw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forwardChoose the right answerarrow_forward8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forward
- Choose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

